- LOGIN
- MemberShip
- 2025-12-22 19:42:14
- ICER of general drugs KRW 15.97 mil for the past 15 yrs
- by Lee, Tak-Sun | translator Kang, Shin-Kook | 2022-12-19 04:35:30
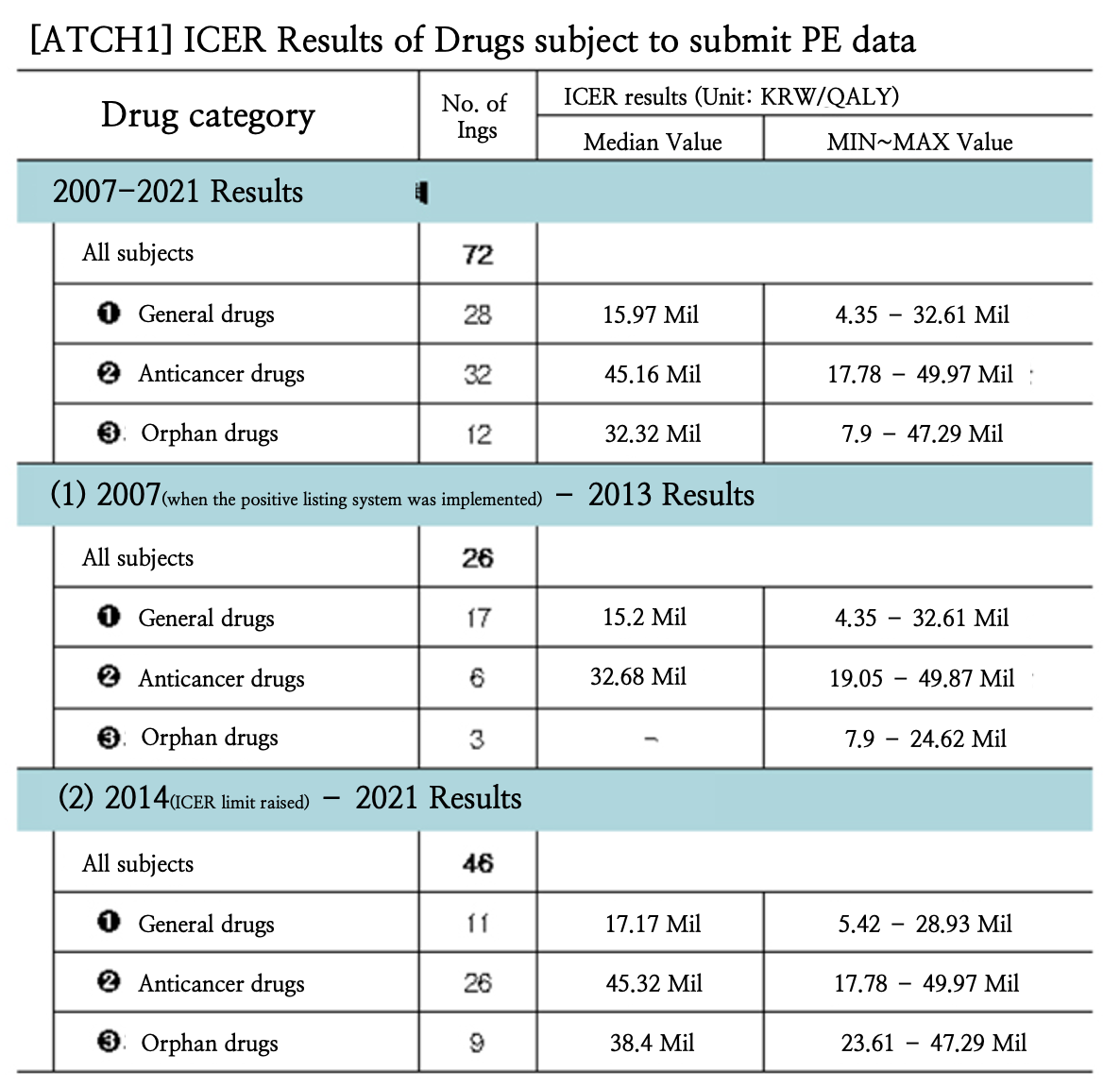
The median ICER (Incremental Cost-Effective Ratio) value of general drugs from 2007 to 2021 was KRW 15.97 million in Korea.
The ICER value of anticancer drugs was KRW 45.16 million, and rare diseases KRW 15.97 million in the same period.
This was the first time that the ICER results were disclosed, and the disclosed results are expected to be useful for pharmaceutical companies that seek to reimburse new drugs.
On the 16th, the Health Insurance Review and Assessment Service announced that it had disclosed the cost-effectiveness evaluation results of drugs that are required to submit pharmacoeconomic evaluation data (ICER) for the past 15 years (2007-2021) since the positive-listing system was first introduced to Korea.
ICER is a value used to evaluate the economic feasibility of a new drug that offers an improved effect and shows the additional cost required per unit of increased effect or efficacy of a new drug compared to its alternative.
Accordingly, a new drug is interpreted as being cost-effective compared to its alternative if the ICER of a certain drug is lower than a certain threshold.
However, instead of using an explicit threshold, Korea flexibly refers to the results of previous deliberations in consideration of the severity and social burden of the disease, its impact on quality of life, and innovativeness.
The disclosure follows the deletion of the 'GDP per capita' standard and the addition of the ‘existing review results' standards in the revised regulations for the ICER threshold in the ‘Detailed Evaluation Standards for Drugs Subject to New Drugs, Etc.’ in September last year.
HIRA explained that the revision specifies the use of the alternative reference value that is used in Korea, as Korea does not use an explicit threshold value.
After the initial disclosure this year, HIRA plans to disclose the 5-year ICER data every December, but in consideration of the number of ingredients in each drug category each period to prevent specifying the evaluation results of individual drugs.
The ingredients subject to disclosure are ingredients deemed cost-effective by the Drug Reimbursement Evaluation Committee and evaluated for reimbursement.
Only for 2022, all data from 2007 to 2021 were disclosed at once.
Also, the evaluation results from 2007 to 2013 and 2014 to 2021 were separately disclosed in consideration of the major policy changes that had been made in 2014, such as the ▲increased ICER limit to strengthen coverage for severe diseases (from November 2013) and the ▲ implementation of the risk-sharing system (from December 2013), etc.
HIRA explained that the data disclosed are divided into three categories: general drugs, anticancer drugs, and rare disease drugs, and the number of ingredients and cost-effectiveness evaluation results for each category are disclosed.
In the case of anticancer and rare disease drugs, the classification is made according to the classifications made during DREC evaluations, and all other drugs are included in the general drug category.
The number of ingredients was calculated based on the results of the cost-effectiveness analysis and subject ingredients, and HIRA will be disclosing the median, minimum, and maximum values based on drug category.
The ICER results of drugs that submitted data for economic evaluations showed that the median ICER of generic drugs from 2007 to 2021 was KRW 15.97 million.
Also, the ICER value of anticancer drugs was KRW 45.16 million, and rare disease drugs KRW 32.32 million.
The ICER results of drugs subject to PE data submissions can be found on HIRA’s webpage.
Mi-Young Yoo, Deputy Minister of HIRA's Pharmaceutical Benefits Management Department, said, “The ICER results that were disclosed this time are meaningful as this is the first time the data had been disclosed since the introduction of the positive-listing system, and the annual regular disclosure of the data is expected to be used as an alternative reference value related to ICER.” Yoo added, “However, in evaluating the reimbursement adequacy of drugs, not only the ▲cost-effectiveness from PE evaluation results, but also the ▲clinical effectiveness, and ▲its impact on NHI finances, are comprehensively considered.
Korea does not use an explicit ICER threshold value; it rather evaluates the value in consideration of the uncertainties based on the results of the sensitivity analysis in addition to the basic analysis results.
Therefore, we ask people to play caution in interpreting the published cost-effectiveness evaluation results.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










