- LOGIN
- MemberShip
- 2025-12-23 06:59:14
- 'Lixiana' dominates the DOAC market in KOR
- by Kim, Jin-Gu | translator Kang, Shin-Kook | 2024-05-09 05:53:02

BMS’s 'Eliquis (apixaban)' sales have recently experienced a slowdown, and Bayer’s 'Xarelto (rivaroxaban)' prescription performance significantly dropped after the generic launch.
The sales of Xarelto generic nearly chased the original product three years after its launch.
The pharmaceutical industry eyes on the substance expiry of Eliquis this September.
Eliquis generics that withdrew from the market after the Supreme Court’s ruling last 2021 may re-enter the market around the time of patent expiry, and a significant shift in the market is expected.
Lixiana, 9% up in one year… Eliquis growth has been stalled According to medical market research firm UBIST, the market of DOAC treatment outpatient prescriptions in Q1 in South Korea amounted to KRW 62.9 billion, up 6% over a year compared to KRW 59.3 billion in Q1 last year.
The mechanism of action of DOAC treatment is based on directly affecting the blood coagulation factor, thereby preventing blood clots.
It is expanding its usage as it replaces warfarin, which prevents vitamin K metabolism.
In South Korea, Xarelto was approved in 2009, followed by Pradaxa and Eliquis in 2011.
Pradaxa and Eliquis were also approved in 2015.
When the product was launched, it was called New Oral Anti-Coagulant (NOAC).
Ten years after its launch, the term was changed to Direct Oral Anti-Coagulant (DOAC) to indicate that the product directly affects the coagulation factor.
Four original products have seen a shift in the market.
The top-selling product, Lixiana, is strengthening its position as the market leader.
Prescription sales of Lixiana in Q1 of last year were KRW 27.7 billion, up 5% over a year compared to KRW 25.5 billion in Q1 of last year.
Although it was launched late, its prescription performance grew fast, reaching the top after 2019.
Last year, Lixiana became the first DOAC product to reach annual prescription sales of KRW 100 billion.
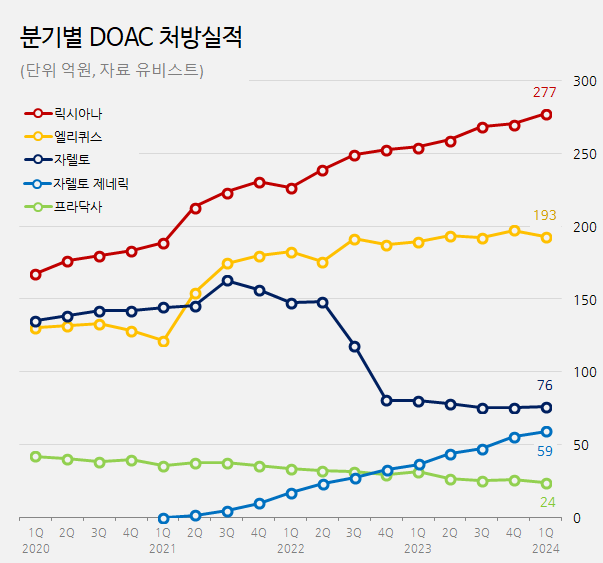
Eliquis prescription sales have seen a 2% growth, increasing from KRW 19 billion in Q1 of last year to KRW 19.3 billion in Q1 of this year.
After Q3 of 2022, Eliquis prescription sales remain around KRW 19 billion.
As Lixiana sales grew fast while Eliquis sales stalled, the sales gap between the two products is widening.
The difference in the prescription sales between these two products in Q1 of this year amounted to KRW 8.5 billion.
It continues to widen every year, with a difference of KRW 4.4 billion in Q1 of 2022 and KRW 6.5 billion in Q1 of 2023.
Low Xarelto generics sales… Riroxban>Riroxia>Rivoxaban Over the last two years, Xarelto has experienced a significant decrease in prescription sales among the major DOAC products.
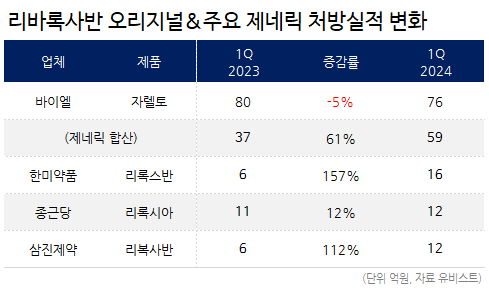
Xarelto’s prescription sales in Q1 this year amounted to KRW 7.6 billion, down 5% compared to KRW 8 billion in Q1 last year.
Its prescription sales declined by almost half over the past two years compared to KRW 14.8 billion in Q1 of 2022.
Xarelto’s prescription sales decline is likely due to its patent expiration and generic release that followed.
Xarelto generic was launched first in Q2 of 2021.
Xarelto’s prescription sales decline is likely due to its patent expiration and generic release that followed.
Xarelto generic was launched first in Q2 of 2021.
In Q3 of 2022, the stoppage of reducing drug prices for Xarelto due to an administrative suit was lifted.
When the pricing of Xarelto decreased, prescription sales started to decline.
Eventually, the presence of Xarelto generics increased while the sales of the original prescription gradually decreased.
Xarelto generics have gradually increased their prescription sales, nearly catching up with those of the original products.
As of Q1, the net prescription sales of generic amounted to KRW 5.9 billion, up 61% over a year compared to KRW 3.7 billion in Q1 of last year.
According to Q1 prescription performance report, Hanmi Pharm’s 'Riroxban' generated KRW 1.6 billion, CKD Pharm’s 'Riroxia' generated KRW 1.2 billion, and Samjin Pharmaceutical’s 'Rivoxaban' generated KRW 1.2 billion.
In Q1, Pradaxa prescription sales amounted to KRW 2.4 billion, down 25% year over year.
Eliquis’s substance patent set to expire this September…generic re-entry predicted The future variable in this market is the expiration of Eliquis' patent.
Eliquis' substance patent expires on September 9th of this year.
Generic manufacturers are waiting for the expiry of the substance patent, with Eliquis' formulation patent set to expire in 2031 after being invalidated.
Generic companies won both the first and second trials related to substance patents in 2018 and 2019, respectively.
After June 2019, Eliquis generic products were subsequently launched and have since been steadily growing in prescription sales.
However, the circumstances changed when the Supreme Court ruled on the reversal of the original decision in April 2021.
Generic companies have retrieved their products that could potentially infringe on the patent.
On the contrary, Eliquis successfully regained its dominant position in the market for DOAC treatments containing the apixaban ingredient.
With the substance patent set to expire this September, a flood of Eliquis generics is possible.
Major generic manufacturers have experience releasing their products and are expected to expand their influence in the market quickly.
On the other hand, it is expected that Eliquis will experience a sharp decline in prescription performance following the release of generics.
From BMS's perspective, there is no justification for delaying the government's price reduction decision following the generic reimbursement listing.
Due to this, the pharmaceutical industry anticipates that Xarelto will further strengthen its dominant position in the DOAC market.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










