- LOGIN
- MemberShip
- 2025-12-23 07:02:02
- Clinical trial failures…novel PD drugs face challenges
- by Son, Hyung-Min | translator Kang, Shin-Kook | 2024-05-23 05:48:42
Novel candidates for Parkinson’s disease that have gathered the industry’s attention are repeatedly failing to prove effectiveness in clinical trials.
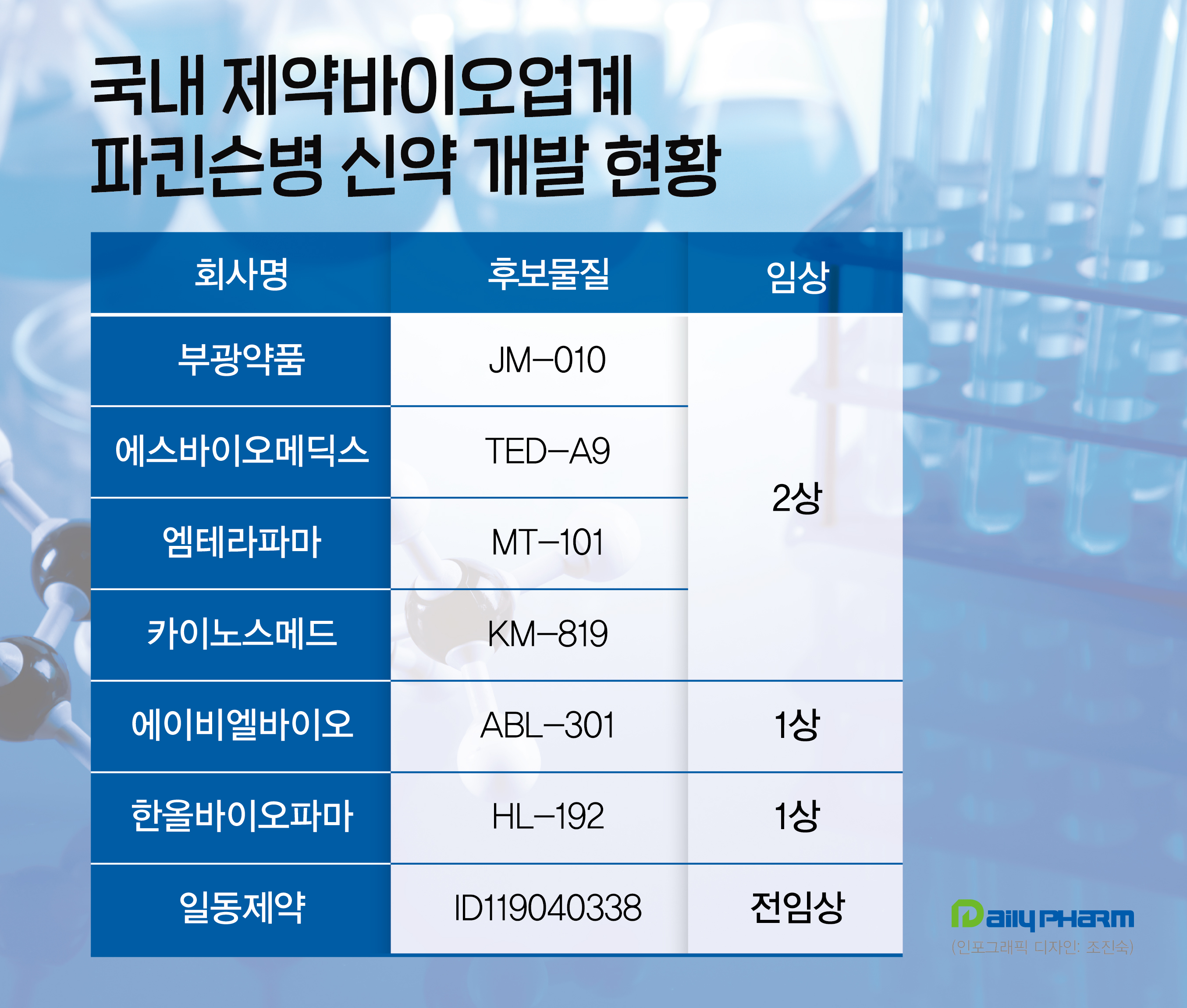
Bukwang Pharmaceutical was developing novel drugs for Parkinson’s disease but failed to prove its efficacy in Europe phase 2 clinical trials.
D&D Pharmatech and Peptron also failed in phase 2 clinical trials.
U.S.
Amneal Pharmaceuticals and AbbVie also faced hurdles from the U.S.
FDA.
Despite of these circumstances, pharmaceutical companies continue to develop novel drugs for Parkinson’s disease and work to turn around failing history.

Contera Pharma specializes in developing neurological disease treatments and was acquired by Bukwang Pharmaceutical in 2014.
JM-010 targets Parkinson’s disease’s dyskinesia treatment.
It has been developed to lessen involuntary movements of arms, legs, and face in Parkinson’s disease patients.
This clinical trial, named Astoria, evaluated the efficacy and safety of JM-010 by administering either JM-010 or placebo in patients with Parkinson’s disease experiencing dyskinesia.
The study was conducted at 38 clinical sites across Europe and Asia, including Germany, France, Italy, Spain, Slovakia, and South Korea, and involved 38 patients with Parkinson's disease.
The clinical trial included patients who experienced dyskinesia as a side effect of levodopa, which is approved for the treatment of Parkinson's disease.
The primary endpoint was the result of dyskinesia reduction, which was evaluated by the Unified Dyskinesia Rating Scale (UDysRS) at 12 weeks.
The clinical results demonstrated that the total scores of UDysRS in the low-dose and high-dose groups of JM-010 were reduced by 0.3 points and 4.2 points, respectively, but the values were not statistically significant.
For the safety profile, two doses of JM-010 have shown good tolerability, and no adverse reactions have been found in the clinical trial.
Contera Pharma is also conducting additional analysis of Astoria’s complete clinical data, including secondary endpoints.
The company will disclose the complete clinical results at a major conference.
D&D Pharmatech·Peptron fail at phase 2 trials…AbbVie rejected by FDA Clinical trials for Parkinson's disease treatment have failed before.
Korean biotech ventures like D&D Pharmatech and Peptron have also experienced challenges in proving efficacy in clinical trials.
D&D Pharmatech's NLY01, a glucagon-like peptide-1 (GLP-1) agonist mechanism, failed to demonstrate efficacy in a phase 2 clinical trial in 2020.
After 36 weeks of administration, it did not show statistically significant improvement in symptom alleviation compared to the placebo group which was set as the primary endpoint parameter.
In detail, at 24 weeks of administration, a significant difference was found between the NLY01 treatment group and the placebo group.
However, between 24-36 weeks, the placebo group’s symptoms showed more improvements than those of the NLY01 group.
Peptron also failed to secure the efficacy of ‘PT320,’ a Parkinson’s disease candidate drug, in a phase 2 trial in South Korea, which was disclosed in 2022.
The PT320 2.0 mg group’s UPDRS part 3 score, which evaluated Parkinson’s disease movement symptoms and was used as the primary endpoint, was not different from the placebo group’s.
The PT320 2.5㎎ treatment group showed symptom improvements but failed to demonstrate statistical significance.
AbbVie failed to receive FDA approval last year.
ABBV-951, under development by AbbVie, has shown significant improvement in the onset time of efficacy compared to Duodopa (active ingredients: carbidopa·levodopa) used as a treatment for Parkinson's disease in phase 3 clinical trials, without exhibiting persistent dyskinesia.
When taken once daily, this medication's effects can last 24 hours.
However, the FDA has requested further clarification on using the adjunctive exercise device with ABBV-951 and has thus rejected approval.
Novel drugs for Parkinson’s disease have failed multiple times in the past…development continues Despite past failures, global pharmaceutical and pharmaceutical and biotech companies in South Korea continue to conduct clinical trials.
Amneal Pharmaceuticals’s IPX203 is an oral formulation of levodopa and carbidopa extended-release capsules.
Levodopa addresses dopamine shortage, which has been pointed out as the primary cause of Parkinson’s disease.
Carbidopa is used as a combination therapy because it can inhibit the conversion of levodopa to dopamine in peripheral nerves.
Last year, the FDA found scientific evidence of levodopa’s safety based on pharmacokinetics research last year.
However, the FDA requested additional information on Carbidopa due to inadequate proof.
The company plans to develop IPX203, which offers longer treatment effects with a lower dose than the conventional formulation.
AbbVie plans to receive approval through an additional novel drug candidate in addition to ABB-951.
AbbVie is conducting a phase 3 trial through Cerevel Therapeutics, which recently disclosed the top-line results of Tavapadon’s phase 3 trials.
Tavapadon is a novel drug candidate for Parkinson’s disease.
It is designed as a dopamine D1/D5 receptor partial agonist taken orally once daily to balance motor control activity and drug efficacy.
The clinical trial evaluated the efficacy and safety of Tavapadon as a levodopa adjuvant therapy in 507 Parkinson’s patients.
Clinical results showed that during the 27-week trial period, the Tavapadon treatment group had a longer duration of Parkinson’s disease remission than the placebo group.
AbbVie plans to share additional data from monotherapy trials of TEMPO-1 and TEMPO-2 by the end of this year.
The novel drug development for Parkinson's disease continues in the pharmaceutical and biotech industry in South Korea.
S.Biomedics is working on stem cell therapy TED-A9, Mthera Pharma is developing the natural drug MT-101, and Kainos Medicine is targeting the Fas Associated Factor 1 (FAF1) protein with KM-819.
Additionally, ABL Bio is working on a dual antibody, ABL-301.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










