- LOGIN
- MemberShip
- 2025-12-23 05:17:51
- Global pharmaceutical companies acquire radiopharmaceuticals
- by Son, Hyung-Min | translator Kang, Shin-Kook | 2024-05-28 05:52:03
The high interest in radiopharmaceuticals has led to investments by pharmaceutical companies.
Last year and this year, Lily invested KRW 3.5 trillion in a company developing radiopharmaceuticals.
Global pharmaceutical companies, including AstraZeneca, Novartis, and BMS, have acquired companies developing radiopharmaceuticals and entered the market.
In South Korea, FutureChem and Dong-A ST are also developing radiopharmaceuticals and exploring opportunities for technology transfer and commercialization potential.
Radiopharmaceuticals work by delivering radioactive isotopes into the body, and then the isotopes release radio waves when reaching cancer cells, destroying cancer organs.
Radiopharmaceuticals are divided up by diagnosis and treatment markets, with the diagnosis market having over 90%.
The major pharmaceutical industry is focused on the radiopharmaceuticals diagnosis market and the treatment market, which represents a blue ocean opportunity.
According to industry sources on May 28th, Lily signed a co-development deal with Aktis Oncology, a company specializing in radiopharmaceuticals.
Through this contract, Lily has acquired the right to commercialize a part of Aktis’ developing products for diagnosis.
Lily will pay Aktis up to US$1.1 billion as it achieves future milestones, including a $60 million upfront payment.
Following the acquisition of POINT Biopharma and the development of biopharmaceuticals with Aktis, Lily demonstrated a focus on the industry.
Lily invested US$1.4 billion (approximately KRW 2 trillion) in acquiring POINT Biopharma’s PNT2022, a prostate cancer treatment candidate, and PNT2003, a neuroendocrine tumor treatment candidate.
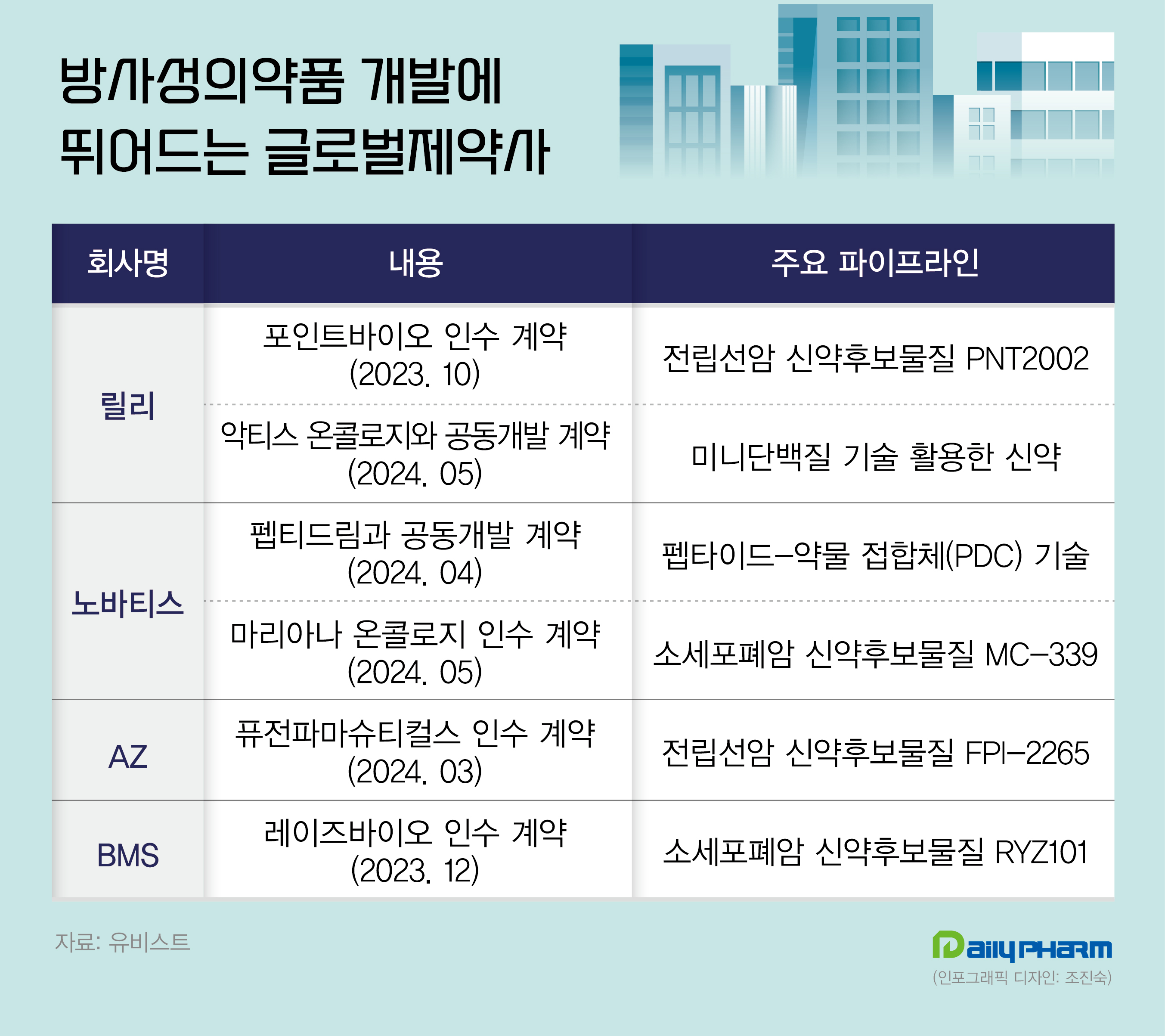
Novartis also completed the acquisition of a company specializing in developing radiopharmaceuticals.
Novartis succeeded in acquiring Mariana Oncology of the United States.
The contract size was up to US$1.75 billion.
Through this acquisition, Novartis secured MC-339, an actinium-based radioligand therapy (RLT).
MC-339 is under clinical trials for indication in small-cell lung cancer.
Radioligand is a treatment that conjugates therapeutic radioisotope with a targeted ligand.
When the therapeutic radioisotope binds to targeted cells, the radioisotope is released, inhibiting the cancer cell growth.
Novartis plans to expand its radiopharmaceuticals pipeline.
Last month, Novartis signed a co-development agreement with Japan’s pharmaceutical company PeptiDream.
PeptiDream has PDC (peptide-drug conjugate) technology, which conjugates a peptide to radionucleotides.
In addition to having prostate cancer treatment Pluvicto and neuroendocrine tumor treatment Lutathera, Novartis aims to secure additional novel drugs.
AstraZeneca and BMS also initiated radiopharmaceuticals development.
In March last year, AstraZeneca acquired Fusion Pharmaceuticals, a Canadian company developing radiopharmaceuticals.
AstraZeneca secured FPI-2265, a novel candidate for treating metastatic prostate cancer.
FPI-2265, targeting prostate-specific membrane antigen (PSMA), is under phase 2 clinical trials.
FPI-2068, a EGFR-cMET targeting radioconjugate, also entered a Phase 1 study.
In December, BMS acquired RayzeBio, a company specializing in radiopharmaceuticals, for approximately US$4.1 billion.
RayzeBio is developing radiopharmaceuticals that target a wide range of solid cancers, including gastroenteropancreatic neuroendocrine tumors (GEP-NET).
Its primary pipeline includes GEP-NET, RYZ101, which targets somatostatin receptor 2 (SSRT2) overexpressed in small-cell lung cancer, and RYZ801, targeting glypican-3 (GPC3).
Attention↑ in South Korea…FutureChem entered a Phase 2a study As global pharmaceutical companies show high interest in radiopharmaceuticals, opportunities for domestic biopharmaceutical companies to transfer technology may be on the horizon.
Currently, FutureChem is evaluated to be at the foremost stage of development.
This month, FutureChem initiated the first administration of FC705, targeting castration-resistant prostate cancer (CRPC), in a patient as part of its Phase 2a study in the United States.
FC705 is a radiopharmaceutical targeting PSMA, which is overexpressed in prostate cancer cell surfaces.
This treatment is based on a PSMA-binding peptide conjugated with a therapeutic radioisotope, which kills the cancer cells.
In Phase 1 clinical trials, all the patients treated with FC705 met overall response rates (ORR) and disease control rates (DCR).
FutureChem is conducting a Phase 2 trial in South Korea, in addition to its U.S.
clinical trials and is under discussion for technology transfer with China.
AbTis, a Dong-A ST’s subsidiary, will start novel radiopharmaceuticals development with CellBion.
Last month, two companies signed a co-development agreement.
Utilizing AbTis’ AbClick, a linker platform technology, and CellBion’s linker technology of radiopharmaceuticals, they plan to develop a novel Antibody-Radionuclide Conjugate (ARC) targeting gastric cancer and pancreatic cancer.
AbTis plans to use Ac-225, a strong therapeutic radioisotope, and cooperate in clinical, production, and commercialization.
SK Biopharmaceuticals designated radiopharmaceuticals as one of three new modalities and aims to enter clinical trials within three years.
In September last year, SK Biopharmaceuticals signed a business agreement with the Korea Institute of Radiological & Medical Sciences (KIRAMS) for novel drug study, clinical development utilizing actinium-225 (Ac-225), the key ingredient of radiopharmaceuticals, and constructing related facilities.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










