- LOGIN
- MemberShip
- 2025-12-23 01:49:42
- Eliquis generics will soon return to market
- by Kim, Jin-Gu | translator Kang, Shin-Kook | 2024-07-03 05:50:35
As such, generic versions of Eliquis, which were withdrawn from the market in 2021 due to the Supreme Court's reversed decision, will re-enter the market thereafter.
The return of generics is expected to once again shake up the DOAC market, which includes Eliquis.
In addition, the first generic exclusivity of Hanmi Pharmaceutical's Monterizine (montelukast-levocetirizine), Eisai’s Fycompa (perampanel), and Dong-A ST’s Jublia (efinaconazole) will expire in the second half of this year.
This raises the possibility of further entry of generics upon the expiry of the preferential marketing period.
Eliquis generics withdrawn by Supreme Court ruling will reenter after September According to the Korean Intellectual Property Office, 97 patents for 43 products will expire in the second half of this year alone.
The most notable product among those is BMS’s Eliquis.
Its product patent expires on September 9th.
Except for the product patent, the rest of the patents have been invalidated by generic companies.
The generic companies planned to relaunch the product in September to coincide with the expiry of the product patent.
The companies have launched Eliquis generics since June 2019.
However, the companies completely withdrew from the market nearly 2 years later in April 2021.
Their products are returning to the market after more than 3 years.
Their entry and withdrawal from the market was decided upon by the 1st to 3rd trial results of the patent suit.
The generic companies won the first and second rounds of the product patent litigations in 2018 and 2019, based on which the companies launched their products.
The generics quickly gained influence in the market, generating nearly KRW 10 billion in prescription sales in their second year.
In Q1 2021, just before they were withdrawn from the market, the market share of apixaban-based DOACs reached 23%.
But the tables turned in April 2021 when the Supreme Court reversed and remanded the trial court's decision.
The companies pulled the infringing products from the market.
Eliquis succeeded in regaining its unrivaled position in the apixaban-based DOAC market.
During that period, Eliquis’s sales continued to grow steadily.
Prescriptions for Eliquis jumped from KRW 52.3 billion in 2020 to KRW 63.1 billion in 2021, the year the generics were withdrawn.
The prescription volume increased further to KRW 73.7 billion in 2022 and KRW 77.3 billion last year.
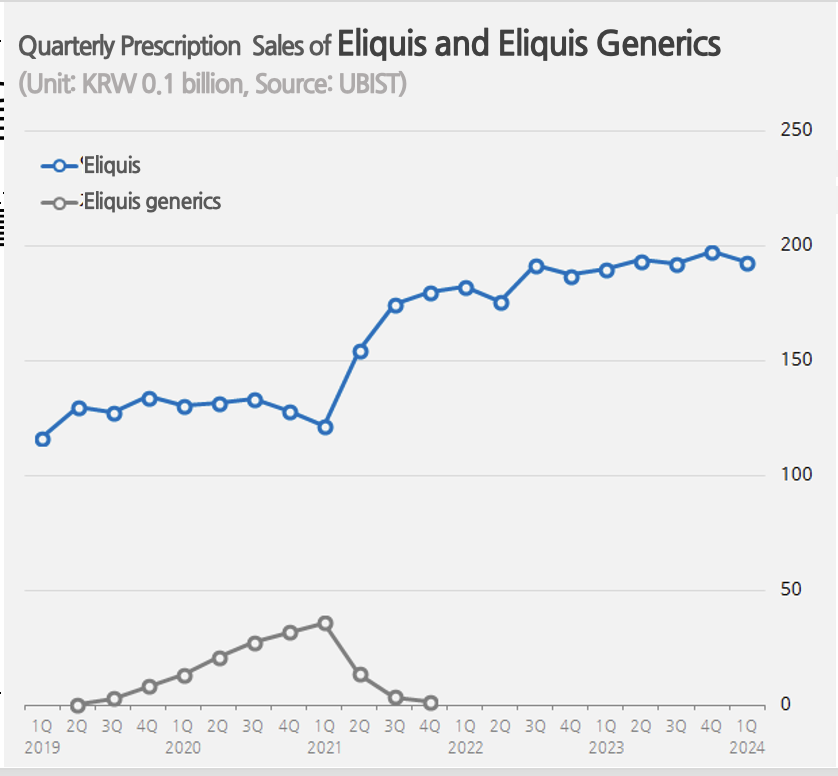
In the past, Eliquis generics have quickly gained market share upon launch.
In addition, they have secured plenty of new clients, selling generics of Xarelto (rivaroxaban), a drug in the same class whose patent expired before Eliquis.
A total of 49 companies have authorized generics of Eliquis.
14 of them were selling the product before it was pulled from the market.
The industry expectation is that around 15 of them will re-enter the market after September.
In addition, patents for AstraZeneca's ovarian and breast cancer drug Lynparza (olaparib), Celgene's multiple myeloma drug Revlimid (lenalidomide), and Takeda Pharmaceuticals’ gastroesophageal reflux disease drug Dexilant DR (dexlansoprazole) will expire in the second half of the year.
In the case of Revlimid, generic companies have already evaded the crystalline patent, which expires in September this year.
Its product patent expired in 2017, and 4 companies had received approval for their generics then and have been selling generics.
In the case of Dexilant DR, 6 companies, including Yuhan Corp, succeeded in avoiding all the remaining patents, including the formulation patent, which expires in July, and 1 generic has already been approved.
In the case of Lynparza, one patent expires on the 23rd of this month.
However, no company has yet challenged the development of Lynparza generics.
First generic exclusivity of Monterizine-Jublia expires...opens the door for generic competition The exclusive sales period of major generics that have entered the market with a first generic exclusivity marketing authorization (first generic exclusivity) will also expire in the second half of this year.
After the first generic exclusivity period expires, likely, latecomer generics will likely also enter the market.
the first generic exclusivity period for Hanmi Pharmaceutical's asthma drug Monterizine expired on the first of this month.
Monterizine’s patent was challenged by 20 companies, including Han Wha Pharm.
The companies won the trial on 4 formulation patents last September and were granted a first 9-month generic exclusivity period.
Within 9 months of launch, the companies won nearly 20% share in the montelukast+levocetirizine combination market.
In Q1 this year, prescriptions for Hanmi Pharmaceutical's Monterizine amounted to KRW 4.3 billion, while the combined prescriptions for its generics amounted to KRW 1.1 billion.
However, it is understood that there have been no follow-up actions made for the development of Monterizine generics thereafter.
Industry analysts say this is because the market size of Monterizine is not large, around KRW 15 billion a year, and competition has been fierce, with as many as 20 companies having received the first generic exclusivity rights.
On the 13th of this month, the distribution period for Eisai’s Fycompa generics for epilepsy will expire.
Myung-in Pharm and Whan In Pharm successfully challenged and evaded Fycompa’s patent in 2020.
Since October last year, Whan In Pharm’s Peranel Tab and Myung In Pharmaceuticals’s Pericompa Tab have been available as generic versions of Fycompa.
However, the performance of the two generics during the marketing period has not been good.
From the fourth quarter of last year to Q1 this year, the cumulative sales of Peranel and Pericompa were only around KRW 20 million each, compared to the KRW 2.2 billion earned by the original product during the same period.
This is due to the high preference for original drugs in the CNS area.
Dong-A ST’s nail fungus treatment Jublia will also expire in November.
16 companies challenged Jublia’s patent, and Daewoong Pharmaceutical and Dongwha Pharmaceutical received the first generic exclusivity rights to market its generics.
Dongwha Pharmaceutical later handed over the license to Huons.
So the other companies that challenged Jublia’s patent but did not receive the first generic exclusivity are expected to join in the generic competition.
Given the summer peak in sales for nail fungus (onychomycosis) treatments, the companies may well join the competition next spring.
Jublia’s sales last year were KRW 31.8 billion.
This is up slightly from the KRW 31.4 billion in 2022.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










