- LOGIN
- MemberShip
- 2025-12-22 22:34:16
- Nabota accounts for 24% of Daewoong’s ETC sales
- by Chon, Seung-Hyun | translator Kang, Shin-Kook | 2024-08-13 05:47:56
Daewoong Pharmaceutical's botulinum toxin 'Nabota' continued its upward sales trend.
The company's quarterly revenue exceeded KRW 50 billion for the first time, led by the company’s growth in overseas markets.
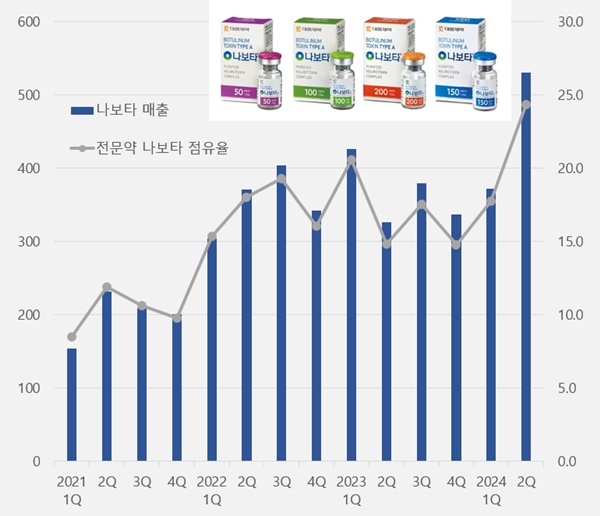
Nabota’s share of Daewoong's specialty drug sales reached nearly 25 percent, driving the company's performance.
According to Daewoong Pharmaceutical on the 12th, Nabota generated sales of KRW 53.1 billion in Q2, up 62.4% year-on-year.
This is the first time since its launch that the drug’s quarterly sales exceeded KRW 50 billion, surpassing the previous record of KRW 42.6 billion set in Q1 last year in less than a year.
Nabota’s sales increased 74.7% in 2 years, compared to sales of KRW 30.4 billion in Q1 2022.
market continued to rise steadily.
Daewoong Pharmaceutical's sales partner for Nabota, Evolus, reported sales of USD 66.9 million (approximately KRW 92 billion) in Q2, up 35.6% from the USD 49.35 million made year-on-year.
This marks the fifth consecutive quarter Evolus renewed its revenue record since the company reported USD 49 million in Q2 last year.
Nabota's export performance has begun to surge since the closure of its strain theft lawsuit with Medytox, which began in 2019, and the established trust in Nabota based on the accumulated U.S.
experience.
In February 2021, Medytox entered into a settlement agreement with Daewoong Pharmaceutical's U.S.
partners Evolus and AbbVie for the sale of Nabota (U.S.
trade name Jeuveau) in the United States.
Under the agreement, Medytox and AbbVie received the rights to continue marketing and distributing Jeuveau in the U.S.
for certain payments from Evolus.
Evolus is also looking to expand into the European market, having launched Nabota in Spain in June.
In Europe, Nabota is currently available under the brand name Nuceiva and is now available in the U.K., Germany, Austria, Italy, and the U.S., in addition to Spain.
In June, the company received marketing authorization for Nabota from Argentina's National Administration of Drugs, Food and Medical Devices (ANMAT), accelerating its international expansion as well.
Nabota will be launched in Argentina under the brand name Clodew in Q4 this year through Daewoong’s local partner Oxapharma.
Daewoong Pharmaceutical currently has botulinum toxin products licensed in more than 70 countries worldwide and partnerships in more than 80 countries.
As a result, Nabota's presence in Daewoong's sales is also gradually expanding.
In Q2 last year, Nabota’s sales accounted for 24.4% of Daewoong's KRW 218 billion in specialty drug sales, the largest share ever.
Among the products developed and sold by Daewoong Pharmaceutical, Nabota has generated the largest sales volume.
Daewoong's sales of specialty drugs increased 11.7% in 3 years from KRW 181 billion in Q2 2021, while Nabota’s sales more than tripled in the same period.
Nabota's share of the company's specialty drug sales has nearly tripled in 3 years from 8.5% in Q2 2021.
Nabota’s share in Daewoong Pharmaceutical's specialty drug sales exceeded 10% in Q3 2020 for the first time.
After surpassing 20% in Q1 last year at 20.6%, it remained in the 10% range until Q1 this year.
However, with the surge in Nabota’s sales, the drug accounted for more than a quarter of the company’s specialty drug sales in Q2.
More recently, the company’s new drug Fexclue has also contributed significantly to company sales.
In Q2, sales of Fexuclue more than doubled year-on-year to KRW 33.2 billion.
Fexclue is a potassium-competitive acid blocker (P-CAB) drug that treats GERD.
Fexuclue was approved by the MFDS in December 2021 and began full-scale sales in July 2022 after being listed for reimbursement on Korea’s health insurance benefit list.
Nabota and Fexuclue jointly generated KRW 86.3 billion in sales in Q2, accounting for 39.6% of the company's specialty drug sales.
Daewoong Pharmaceutical set a new performance record thanks to the steep growth of its self-developed drugs.
On a standalone basis, Daewoong Pharmaceutical's Q2 revenue increased 6.0% year-on-year to KRW 325.5 billion, and operating profit increased 37.1% to KRW 49.6 billion.
Both the revenue and operating profit in Q2 were the largest in the company's history.
The operating profit margin as a percentage of sales was 15.2%, a significant improvement from the 11.8% it had made in the same period of the previous year, showing high performance.
Daewoong Pharmaceutical's operating profit on a consolidated basis was KRW 42.3 billion in Q2, up 5.6% year-on-year, the largest ever.
Revenue increased 3.0% year-on-year to KRW 360.5 billion, the second-highest amount ever recorded after Q4 of last year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










