- LOGIN
- MemberShip
- 2025-12-22 15:09:28
- Active development of new drugs for myasthenia gravis
- by Son, Hyung Min | translator Hong, Ji Yeon | 2024-11-27 05:50:31
Competition in the pharmaceutical market is heating up to secure myasthenia gravis indication.
UCB's new drug was approved in South Korea, and Janssen finished the phase 3 trial, aiming to acquire regulatory approvals worldwide.
In South Korea, Handok seeks to enter the market with its acquired new drug.
HanAll Biopharma is conducting a Phase 3 trial and investigating the potential of commercialization.
According to industry sources on November 27, UCB's 'Zilbrysq' was approved in South Korea on November 21.
Zilbrysq can be used as an add-on to standard therapies, such as cholinesterase inhibitors, steroids, and immune checkpoint inhibitors.
This therapy is a complement C5 inhibitor and works by inhibiting the complement-mediated damage to the neuromuscular junction (NMJ).
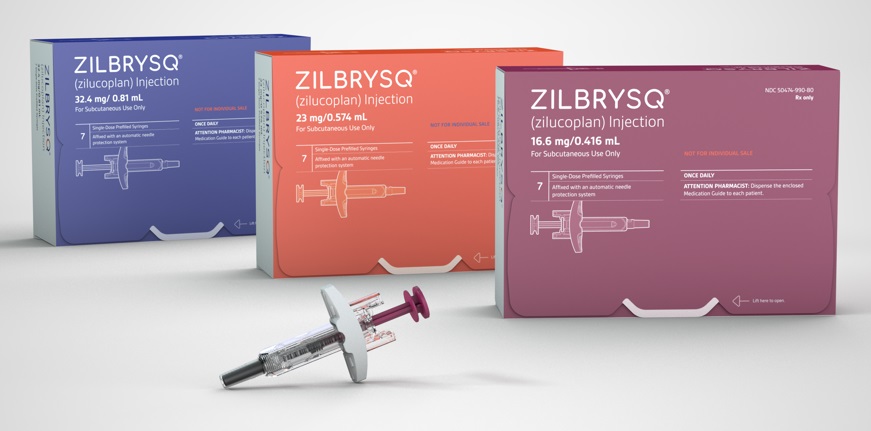
'Ultomiris,' a complement C5 inhibitor used to treat existing myasthenia gravis, is an intravenous injectable that requires administration at hospital visits every 8 weeks.
Generalized myasthenia gravis (gMG) is a chronic autoimmune rare disease in which nerves fail to transmit signals to muscles, leading to skeletal muscle weakness.
This disease induces fluctuating weakness of systemic muscles, affecting daily activities such as standing, swallowing, and breathing.
Zilbrysq's approval is based on the Phase 3 'RAISE' study.
The study evaluated the efficacy and safety of Zilbrysq in 174 adult patients with anti-acetylcholine receptor (AchR) positive gMG.
The primary endpoint was the 'Myasthenia Gravis-Activities of Daily Living (MG-ADL)' for gMG.
Typically, an MG-ADL score of 2 or higher or a Quantitative Myasthenia Gravis (QMG) score of 3 or higher indicates clinically significant improvement.
The clinical results showed that the Zilbrysq group showed a clinically significant reduction by a total score of 4.39 from baseline at 12 weeks compared to the score of 2.30 of the placebo group.
In addition to working on Zilbrysq, UCB is preparing to expand approvals of 'Rystiggo,' UCB's additional treatment for myasthenia gravis, in countries.
Rystiggo obtained the U.S.
Food and Drug Administration (FDA) approval in June 2023 and was also approved in Europe in January of this year.
Janssen has recently completed the Phase 3 trial of 'nipocalimab,' a new drug candidate for myasthenia gravis and the company is preparing to obtain approval.
Janssen applied to obtain approvals from the FDA in August and the European Medicines Agency (EMA) in September.
Nipocalimab works by a novel mechanism that blocks the Fc receptor (FcRn), a protective immunoglobulin G (IgG) receptor.
This mechanism reduces IgG antibodies that cause the disease and inhibits their recycling process.
Nipocalimab binds to FcRn to prevent the degradation of IgG antibodies.
The Phase 3 Vivacity-MG3 study demonstrated the efficacy of nipocalimab in combination with standard therapy compared to the placebo.
The clinical study confirmed a statistically significant result, with nipocalimab recording an MG-ADL score of 4.70.
Also, nipocalimab improved the myasthenia gravis patients' muscular strength and function compared to the placebo.
Handok·HanAll aim to commercialize their treatments with a similar mechanism to nipocalimab
In August.
Handok signed an agreement with argenx, the Belgium-based developer of Vyvgart.
Handok is now responsible for domestic approval registration, applying for reimbursement, and exclusive distribution.
Like nipocalimab, Vyvgarth is a new drug targeting the FcRn.
It has been approved in several countries, including the United States, Europe, the United Kingdom, Israel, and China, for treating adult patients with generalized myasthenia gravis.
The clinical efficacy of Vyvgarth was demonstrated in the Phase 3 clinical trial named ADAPT.
It was shown that Vyvgarth recorded a 68% responder rate based on the MG-ADL scale, significantly higher than the 30% of the placebo.
Additionally, Vyvgarth demonstrated an improved score compared to the placebo group on the QMG scale.
HanAll Biopharma is also developing an FcRn antibody treatment candidate, batoclimab (HL161), as a subcutaneous injectable.
In December 2023, HanAll Biopharma's Chinese partner, Harbour BioMed, secured Phase 3 clinical trial results and submitted an approval application to Chinese regulatory authorities.
Harbour BioMed said that batoclimab improved symptoms in myasthenia gravis patients based on MG-ADL and QMG scales.
HanAll Biopharma is conducting a phase 3 trial for batoclimab in the United States.
HanAll Biopharma's U.S.
partnering company Immunovant finished registering patients for the Phase 3 clinical for myasthenia gravis.
Immunovant plans to present top-line results of the phase 3 clinical trial in the first half of 2025.
HanAll Biopharma and Immunovant plan to investigate the potential of batoclimab for various autoimmune disease indications, including thyroid eye disease and chronic inflammatory demyelinating polyneuropathy.
In addition to developing batoclimab, both companies will develop new drugs for additional myasthenia gravis indication.
HanAll Biopharma and Immunovant are conducting a phase 1 trial of IMVT-1402, a new FcRn antibody drug candidate product.
Unlike conventional FcRn antibody treatments, IMVT-1402 is not known to affect the LDL-cholesterol level.
In the Phase 1 clinical trial, subcutaneous injection of IMVT-1402 and placebo are administered to randomized healthy adults.
Each patient group receives a single-dose or multiple doses with a dose-escalation.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










