- LOGIN
- MemberShip
- 2025-12-22 03:55:31
- Antibody drugs, AI, and platform companies go public
- by Cha, Jihyun | translator Alice Kang | 2025-02-25 05:56:45
Domestic pharmaceutical, bio, and healthcare companies are floating IPOs one after another.
Organoid Sciences is the first to challenge the market with its gap technology.
IntoCell plans to submit a securities report as early as next month.
Companies in various fields, including biotech companies that develop new drugs, medical artificial intelligence (AI) companies, and drug platform developers, are also preparing their listings on KOSDAQ.
According to the Financial Supervisory Service on the 24th, Neurophet submitted a preliminary application for a special case for technology and growth listing to the Korea Exchange on the 21st.
Last August, the company received an A rating from the Korea Technology Finance Corporation, and a BBB rating from the Korea Technology Credit Evaluation, both exchange-designated evaluation agencies, about six months after passing technology evaluations.
Neurophet is a developer of AI solutions for imaging brain diseases.
Its flagship products include Neurophet Aqua AD, a software that monitors the therapeutic effects and side effects of dementia drugs, and Neurophet Aqua, an imaging analysis software for brain nerve degeneration.
Neurophet Aqua AD provides all the brain imaging analysis functions required for the administration of dementia drugs by quantitatively analyzing magnetic resonance imaging (MRI) and positron emission tomography (PET) images.
It selects the patient groups who will benefit from the anti-amyloid drug before administration and analyzes the side effects and effects of the treatment process.
From the patient's point of view, it can reduce the risk of treatment side effects, and from the medical staff's point of view, it can reduce the burden of reading images.
As the effectiveness of the treatment can be analyzed, the possibility of successfully developing new drugs and the efficiency of clinical operations can be dramatically improved.
Neurophet Aqua AD was launched in the second half of last year.
Based on this, the company is also continuing to grow its external profile.
In 2023, the company generated sales of KRW 1.6 billion on a separate basis, an increase of 141% from the previous year.
The company is growing rapidly every year, with KRW 45 million in 2020, KRW 100 million in 2021, and KRW 600 million in 2022.
The company explained that the more prescriptions for amyloidosis treatments are made, the more Neurophet products will be used.
Neurophet plans to list a total of 11,476,035 shares, including 2 million shares to be offered to the public.
Mirae Asset Securities is the listing sponsor.
As a result, 3 pharmaceutical, biotech, and healthcare companies have applied for preliminary listing this year alone.
On the 24th of last month, Novelty Nobility submitted a preliminary listing application to the Korea Exchange.
The company received an A grade from two exchange-designated evaluation agencies in July of last year.
Novelty Nobility is an antibody drug development company established in 2017.
It is conducting R&D on anticancer drugs, eye diseases, and autoimmune diseases using the “one-source multi-use” strategy, which applies a single antibody to various modalities.
It has a fully human antibody platform, PREXISE-D, using humanized mice, and a third-generation linker technology, PREXISE-L.
Novelty Nobility plans to list a total of 16,914,564 shares, including 2,208,000 shares to be offered to the public, through this IPO.
The listing sponsor is Shinhan Investment Corp.
G2GBio, a developer of long-acting injectable drugs, also applied for preliminary listing on the KOSDAQ on the 18th.
G2GBio received an A grade from both NICE D&B and Korea Rating & Data in August last year, thus clearing the first hurdle to be listed on the KOSDAQ as a special case for technology and growth.
G2GBio owns the technology to manufacture microparticles as small as micrometers (㎛) called 'InnoLAMP'.
This is a technology that can quickly produce uniform microparticles in large quantities.
This has enabled CJ to secure a pipeline of products including GB-5001/GB-5112, a drug for the treatment of dementia, GB-6002, a drug for the treatment of pain after surgery, and GB-7001, a drug for the treatment of diabetes.
G2GBio is listing a total of 5.128836 million shares, including 665,000 shares to be offered to the public.
Mirae Asset Securities is the listing sponsor.
There are also companies that have begun their IPO process in earnest.
Organoid Sciences will conduct a demand forecast for institutional investors for 5 business days starting on the 7th of next month.
Afterwards, the final offering price will be confirmed on the 17th of the same month, and subscriptions from institutional and general investors will be conducted for 2 days from the 19th to the 20th.
Organoid Sciences has set out to be the first company to be listed with the super gap technology special case.
The Super gap technology special exemption system was newly established by the financial authorities last year, which allows companies in the fields of advanced and strategic technologies that need to be fostered at the national level, such as deep tech and deep science, to be evaluated for technological excellence based on their growth potential in the market.
A company can pass the technology evaluation by receiving an A grade from only one professional evaluation agency designated by the Korea Exchange.
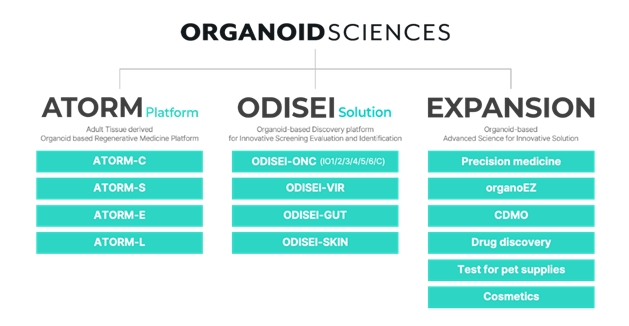
Organoid is a compound word of 'organ' and the suffix 'oid'.
Stem cells or organ-based cells are cultured or recombined into structures similar to organs.
As of the end of last year, Cha Biotech holds a 9.27% stake in Organoidis.
Organoid Sciences is creating a revenue base by commercializing its self-developed platform.
Following the launch of its spatial biology-based genetic analysis platform 'Odyssey' at the end of 2022, it launched 'Organoid' last year, a researcher-oriented organoid culture service.
It is also selling a drug evaluation platform 'ADIO' to pharmaceutical companies.
As a result, the company's consolidated sales, which were around KRW 300 million in 2021, are expected to increase to KRW 1.6 billion by 2023.
Organoid Sciences plans to list a total of 6,494,950 shares, including 1.2 million shares to be offered to the public.
The listing sponsor is Korea Investment Securities.
The offering structure is 100% new shares.
The IPO proceeds will be used to advance the company's technology and global expansion.
IntoCell, a biotech company specializing in antibody-drug conjugates (ADCs), plans to file an IPO registration statement by the end of next month at the earliest.
IntoCell received preliminary approval for listing at the end of August last year and obtained preliminary approval for listing last month.
It took 98 business days from the submission of the preliminary approval for listing to the results of the preliminary review for listing on the Korean exchange.
IntoCell was founded in 2015 by Taekyo Park, co-founder of LigaChem Biosciences.
Park is a bio expert who earned a Bachelor's and Master's degree in Chemistry from Seoul National University and a doctorate in Chemistry from the Massachusetts Institute of Technology (MIT).
He is one of the 7 co-founders of Ligachem Bio, having worked at the LG Life Sciences Research Institute.
As Chief Technology Officer (CTO), he is also the person who laid the foundation for the LigaChem Biosciences’ ADC platform.
IntoCell has strengths in the linker, one of the three elements of ADC: antibody, linker, and drug (payload).
It presents its in-house developed linker platform, OHPAS, as its core competitive advantage.
Linkers are divided into left linkers that attach antibodies and right linkers that attach drugs, and the company has technology specialized in the right linker.
In addition, 'PMT' (Payload Modification Technology) is also a core platform of IntoCell.
It is a technology that minimizes the penetration of highly toxic drugs into normal cells by coating them with a membrane.
This increases the therapeutic index (TI), which is the difference between the dose at which the drug begins to show efficacy and the dose at which side effects appear.
CEO Park completed the development in 2021.
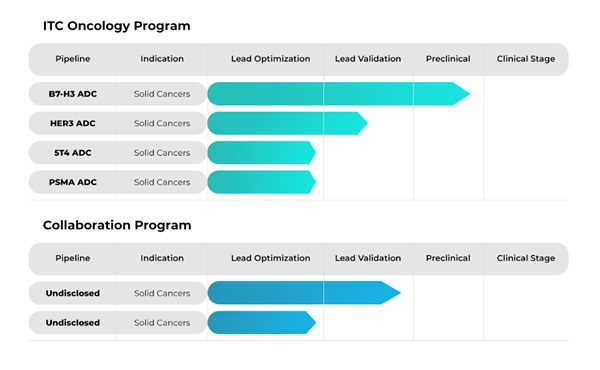
It is expected to secure final preclinical data within this month.
The goal is to submit an IND for Phase I clinical trials in the first half of this year and enter clinical trials within the year.
In addition, IntoCell has a total of 10 pipelines, including lead optimization candidates such as Trop2-ADC, HER2 ISAC, and HER3 ADC.
IntoCell is also making tangible achievements.
Following the technology export of its platform to Swiss ADC Therapeutics in early 2023, it signed a research collaboration agreement (RCA) with Samsung Bioepis at the end of the same year.
The main goal is to develop ADC new drug candidates using the IntoCell platform technology for up to 5 targets.
Samsung Bioepis has received a lot of attention from the industry as it was selected as the first new drug development partner.
In addition, ▲ImmunOncia, ▲Genosco ▲GC Genome ▲Proteina, etc.
are awaiting the results of the examination after submitting a preliminary prospectus to the Korea Exchange.
ImmunOncia and Genosco are also bio-ventures that are highly sought after by investors.
ImmunOncia, a company specializing in the development of immunotherapeutics, is a joint venture established in 2016 by Yuhan Corp and Sorrento Therapeutics of the United States.
At the end of 2023, Yuhan Corp acquired all of Sorrento's shares, holding a 67% stake.
ImmunOncia filed a preliminary listing request in October last year after passing the technical evaluation in April last year.
Genosco filed a preliminary hearing request for a technical special listing in October last year.
Genosco is famous as the original developer of Leclaza, the 31st domestically produced new drug and the first domestically produced new anticancer drug approved by the US Food and Drug Administration (FDA).
In early 2010, the company developed a candidate substance with its parent company, Oscotec, and in 2015, it exported the technology to Yuhan Corp at the pre-clinical stage.
In April last year, Genosco passed the technical evaluation by receiving an AA rating from two professional evaluation agencies.
Genosco is the only new drug developer to have received the highest rating (AA·AA) in the technical evaluation.
Even if the scope is expanded to the pharmaceutical, bio, and healthcare industries, there is only one company, Lunit, a medical artificial intelligence (AI) company.
GC Genome, a genomics analysis subsidiary of the GC Group, submitted a preliminary review request for listing on the KOSDAQ exchange at the end of last year.
It plans to list a total of 22.5 million shares, including 2,944,445 shares to be offered to the public.
Proteina, a protein-protein interaction (PPI) big data analysis company, also applied for a preliminary injunction in November last year.
Proteina is a bio-venture company established in 2015 that uses its own big data technology to help domestic and foreign pharmaceutical companies develop new drugs.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










