- LOGIN
- MemberShip
- 2025-12-22 03:55:30
- Wegovy dominates the obesity drug market
- by Chon, Seung-Hyun | translator Hong, Ji Yeon | 2025-03-13 05:59:22
The introduction of Novo Nordisk's Wegovy has shaken the market substantially.
Since its launch in Q4, Wegovy has captured 60% of the entire market.
Given the introduction of Wegovy, the obesity market expanded to the largest in history.
It also consumed the market for Saxenda, containing the same class of active ingredients as Weogvy.
It is unclear whether such growth will continue because of restricted non-face-to-face medical prescriptions of obesity drugs.
However, the market continues to shake up whenever effective and safe new obesity drugs from multinational companies are launched.
Record sales in 2024 obesity drug market…Wegovy's Q4 sales amounted to KRW 60.3 billion, with 64% market share According to pharmaceutical market research firm IQVIA, on March 10, last year's obesity drug market amounted to KRW 236.3 billion, up 32.8% compared to the previous year.
The obesity drug market continued to set the largest size for seven consecutive years since 2018, exceeding KRW 200 billion for the first time in history.
Last year's growth of the obesity drug market was led by Novo Nordisk's Wegovy.
Wegovy launched in October last year and recorded KRW 60.3 billion in three months.
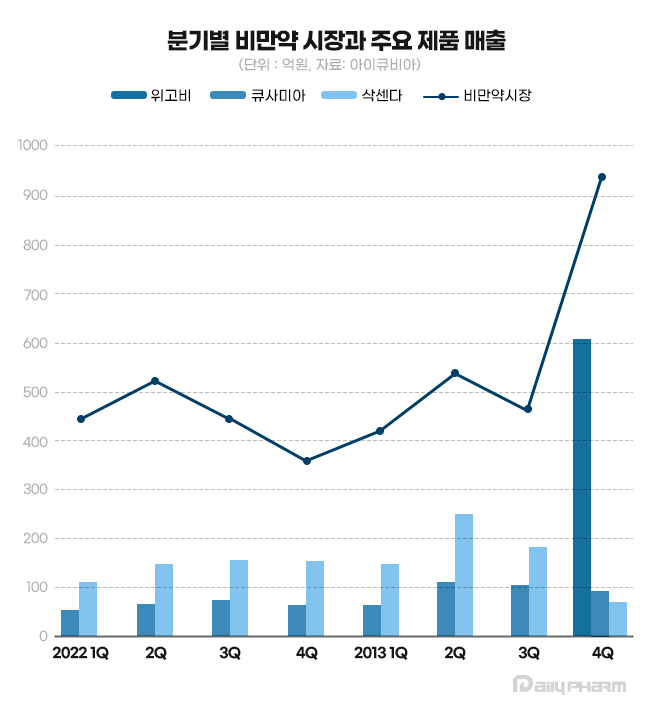
(Legend from left) Wegovy, Qsymia, Saxenda, obesity drug market (unit: KRW 100 million, source: IQVIA) Wegovy received approval from the Ministry of Food and Drug Safety (MFDS) in April 2023.
It is a GLP-1-containing semaglutide that has been shown to reduce glycated hemoglobin.
While conducting clinical trials for GLP-1 diabetes treatment candidates, Novo Nordisk confirmed the effects of lowering patient body weight and developed semaglutide-containing Wegovy as an obesity drug with a once-weekly formulation.
Last year's Q4 obesity market size amounted to KRW 93.8 billion, an expansion of 154.5% Year-over-Year (YoY).
Wegovy accounted for 64.4% of the entire obesity drug market.
Wegovy is trending globally with its outstanding weight loss effects.
Wegovy recorded last year's sales of NOK 58.2 billion (About KRW 11.7 trillion), an increase of 85.7% compared to 2023 sales of NOK 31.3 billion.
Since its launch in the United States, the drug has been sold out with a rapid increase in demand.
Before its official launch, Wegovy was already regarded as a secret weight loss solution among celebrities, including Tesla CEO Elon Musk, and there was a global shortage.
Despite being priced KRW 500,000 higher than the other drugs, Wegovy generated significant interest following its domestic launch and was in short supply.
Due to the introduction of Wegovy, the sales of Saxenda and Qsymia, which previously dominated the obesity drug market, have significantly decreased.
Last year, Saxenda recorded sales of KRW 65.6 billion, down 1.7% from the previous year.
The decrease in sales of Saxenda was seen three years after 2021.
Saxenda reported a significant decline in sales in Q4 of last year.
Saxenda's sales in Q4 of last year amounted to KRW 7.3 billion, down 27.3% YoY.
The sales declined by 78.9% in a quarter from KRW 18.9 billion in Q3 of last year.
Analysis suggests Wegovy, a GLP-1 drug similar to Saxenda, has captured the market for Saxenda.
However, Qsymia's sales have not changed much since the launch of Wegovy.
Qsymia recorded sales of KRW 39.1 billion last year, up 10.1% from the previous year.
In Q4 last year, when Wegovy launched, Qsymia recorded sales of KRW 9.3 billion, down 7.1% from the previous year.
However, it is unclear whether Wegovy will show marked growth this year.
Previously, Wegovy was prescribed through non-face-to-face medical sessions.
As concerns have been raised regarding unrestricted prescription of Wegovy through non-face-to-face medical sessions regardless of weight or obesity status, the health authority discontinued non-face-to-face prescription of obesity drugs as of December 16, 2024.
The obestiy drug market fluctuates whenever new drugs launch…Saxenda has led the market for the past five years The obesity drug market underwent restructuring whenever promising new products are launched.
Products containing sibutramine, which inhibits appetite, sold the most and once dominated the market.
However, it has no longer been in sale due to the risk of cardiovascular side effects since 2010.
The Korean market of obesity drug market was sluggish for a long time.
Once worth a market size of KRW 116.2 billion in 2009, it dropped to KRW 66.7 billion over five years.
Since 2015, the introduction of new products has led to a rebound in the market.
In February 2015, Ildong Pharmaceutical obtained domestic approval for 'Belviq,' which the company acquired from the U.S.-based Arena Pharmaceuticals and led the recovery of the entire market.
Belviq selectively works on the neurotransmitter serotonin receptor, which regulates appetite and emotion, suppressing appetite and increasing meal-related satiety.
It has gained attention for being the new drug approved for weight-loss medication by the U.S.
Food and Drug Administration (FDA) in 13 years.
Kwang-dong Pharm has contributed to the market expansion after launching 'Contrave' in 2016.
Kwang-dong Pharm acquired Contrave from the U.S.-based biotech company Orexigen Therapeutics.
Contrave was approved by the European Medicines Agency (EMA) in 2015, and it is used to manage the weight of adults who are overweight or obese.
After the launching of Belviq and Contrave, the obesity drug market expanded to KRW 92.8 billion and KRW 96.8 billion in 2017 and 2018, respectively.
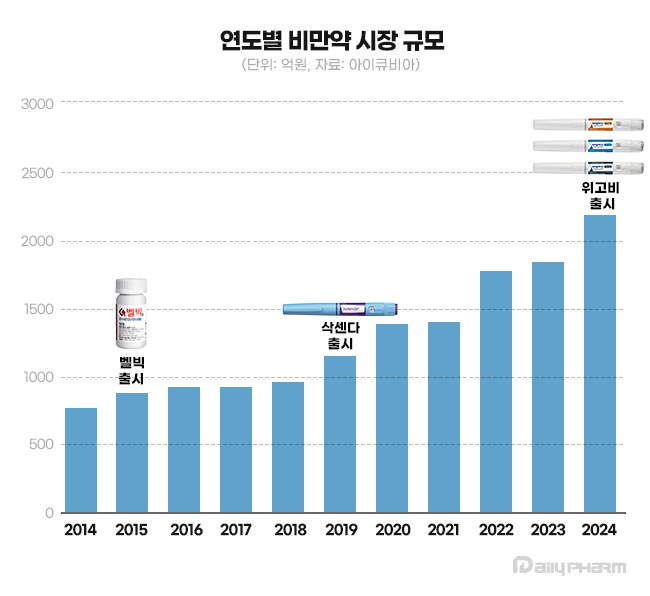
Belviq, Saxenda, and Wegovy were launched in 2015, 2019, and 2024, respectively. Saxenda, launched in Korea in 2018, is the first glucagon-like GLP-1 agonist medication for obesity.
It contains the same ingredient as Victoza (ingredient: liraglutide), which is prescribed to patients with type 2 diabetes but with different methods of administration and dosages.
Saxenda became the top-selling drug in the market after recording sales of KRW 42.6 billion in 2019, just after its launch, and maintained the place for five consecutive years until 2023.
Saxenda recorded sales of KRW 66.8 billion in 2023.
Saxenda took up a 37.5% market share of the obesity market in 2023.
The obesity drug market set a record in 2019 with KRW 134.1 billion in 10 years.
In 2023, it recorded KRW 178 billion, setting a record for five consecutive years.
Qsymia, marketed by Alvogen Korea, has also contributed to the expansion of the obesity drug market.
Launched in late 2019, Qsymia is a combination therapy containing 'phentermine' and 'topiramate.' Alvogen Korea secured domestic sales rights from the U.S.-based VIVUS in 2017.
In late 2019, Alvogen Korea signed a co-promotion agreement with Chong Kun Dang to begin full-scale domestic sales.
Qsymia recorded sales of KRW 35.5 billion in 2023.
The sales of Qsymia were comparable to Saxenda.
Despite oral administration, Qsymia has a relatively small amount of psychotropic drug ingredients, and it has the advantage that it can be prescribed for an extended period.
Alvogen Korea's extensive sales networks in the domestic obesity market, gained from its previous experience selling Furing and Furimin, synergized with Chong Kun Dang's business power to penetrate the market with Qsymia rapidly.
With the launch of Wegovy last year, the obesity drug market underwent another restructuring, and the introduction of Mounjaro and other next-generation obesity treatments is expected to reshape the landscape further.
Eli Lilly’s Mounjaro received approval from the MFDS in June 2023.
Mounjaro, a once-weekly injectable, is a next-generation GLP-1 analog that activates GLP-1 and GIP receptors.
It has been demonstrated that Mounjaro has superior weight loss compared to Wegovy.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










