- LOGIN
- MemberShip
- 2025-12-22 07:22:50
- Ja Q Bo ₩9B, K-CAB ₩8.2B, Fexuclue ₩4.7B
- by Chon, Seung-Hyun | translator Alice Kang | 2025-03-27 05:54:36
Homegrown P-CAB class new drugs for gastroesophageal reflux disease (GERD) have begun to generate export sales.
Onconic Therapeutics’ Ja Q Bo generated significantly more overseas sales than domestic sales due to the effect of its licensing out deals.
HK Inno.N's K-CAB and Daewoong Pharmaceutical's Fexuclue have begun to generate export sales in earnest with their overseas launch.
As P-CAB class new drugs demonstrated their marketability in the domestic market through commercial success, overseas sales are expected to grow with the increase in the number of export countries.
According to the Financial Supervisory Service on the 25th, Onconic Therapeutics' Ja Q Bo generated KRW 14.8 billion in sales last year.
Onconic Therapeutics, which was established in May 2020, is a new drug developer subsidiary of Jeil Pharmaceutical.
Onconic Therapeutics was launched after receiving technology transfer of new drug candidates for gastroesophageal diseases and a new drug candidate for anticancer drugs from Jeil Pharmaceutical.
As of the end of last year, Jeil Pharmaceutical holds a 46.28% stake in Onconic Therapeutics.
Onconic Therapeutics was listed on the KOSDAQ market in December last year.
Onconic Therapeutics completed clinical trials for Ja Q Bo, a P-CAB (potassium-competitive acid blocker) class new drug, and received approval for the drug as the 37th homegrown new drug in April last year.
Antiulcer drugs in the P-CAB class inhibit gastric acid secretion by competitively binding to proton pumps and potassium ions located at the final stage of acid secretion in gastric parietal cells.
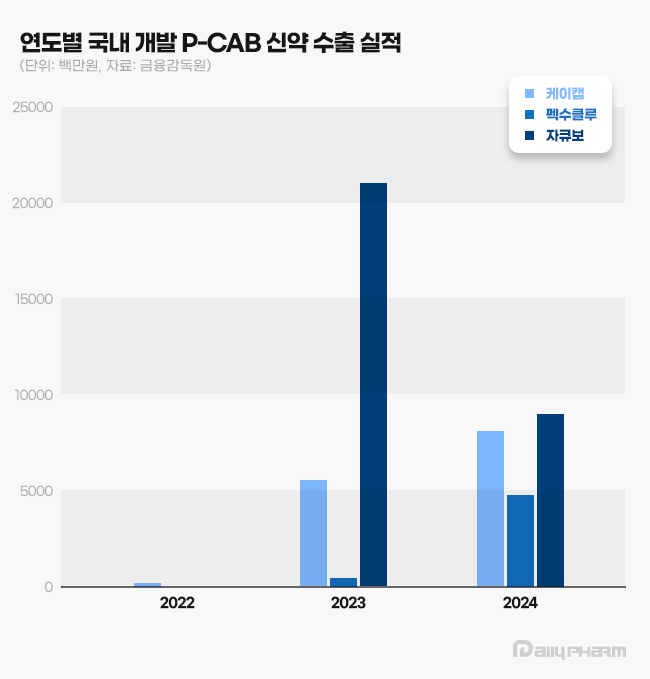
Ja Q Bo began domestic sales in October last year after being listed for reimbursement in the National Health Insurance, recording KRW 5.8 billion in domestic sales.
Ja Q Bo's export performance last year was due to the inflow of milestone payments from the results of the licensing-out agreement.
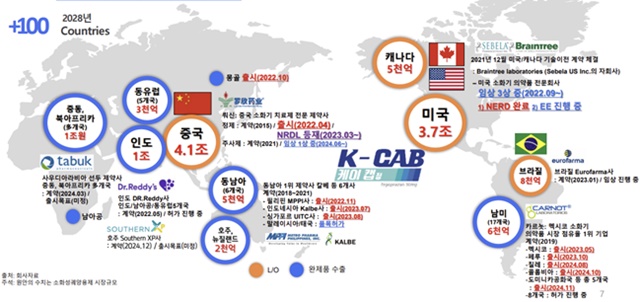
Onconic Therapeutics has signed licensing out deals for Ja Q Bo in 21 countries.
Onconic Therapeutics signed a licensing out deal with the Mexican pharmaceutical company Laboratorios Sanfer in September last year.
The contract is worth up to USD 127.5 million.
Onconic Therapeutics will receive an initial non-refundable upfront payment of USD 15 million and up to USD 112.5 million in milestone payments for development, licensing, and commercialization milestones.
Onconic Therapeutics transferred Ja Q Bo to an Indian company in May last year.
The Indian company has secured exclusive rights to the development, licensing, production, and post-launch commercialization of Ja Q Bo.
The terms of the contract with the other party have not been disclosed.
Ja Q Bo generated KRW 21.1 billion in exports in 2023.
Onconic Therapeutics signed a licensing-out agreement for Ja Q Bo with Livzon Pharmaceutical Group, a Chinese pharmaceutical company, in March 2023.
The contract size is up to USD 127.5 million.
Onconic Therapeutics will receive a USD 15 million non-refundable upfront payment and up to USD 112.5 million in milestone payments for development, licensing, and commercialization.
Onconic Therapeutics generated KRW 19.7 billion in sales in the first quarter of 2023.
Sales of K-CAB, a new P-CAB class drug that first entered the domestic market, and Fexuclue have also been increasing gradually overseas.
HK Inno.N’s K-CAB recorded KRW 8.2 billion in exports last year.
This is the overseas sales of the finished drug, excluding technology fees and milestone payments.
Although the overseas sales share in K-CAB's total sales of KRW 168.9 billion is negligible, the export value is continuously increasing.
K-CAB recorded its first export performance of KRW 900 million in 2022 and KRW 5.5 billion in overseas sales in 2023.
K-CAB was approved as the 30th homegrown new drug in 2018.
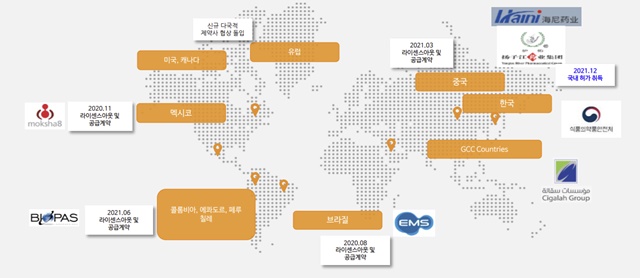
HK Inno.N signed a technology export agreement with the Chinese pharmaceutical company Luoxin in 2015 and has been pushing K-CAB's overseas expansion in earnest.
The contract with Luoxin is conditional on receiving a total of USD 18.5 million in technology fees upon achievement of each stage specified in the contract, including the down payment, clinical development, licensing, and commercialization.
The contract is worth USD 84 million over 10 years, including the product supply price.
HK Inno.N has since signed export contracts for K-CAB with Indonesia, Thailand, the Philippines, Mongolia, Singapore, Vietnam, Malaysia, the United States, and Canada.
In January of last year, it signed a contract with Australian pharmaceutical company Southern XP to export K-CAB to Australia and New Zealand.
K-CAB has been launched in 15 countries.
The countries where K-CAB has been launched include China, the Philippines, Mongolia, Mexico, Indonesia, Singapore, Peru, Chile, the Dominican Republic, Nicaragua, Honduras, Guatemala, El Salvador, and Colombia. K-CAB's export performance has been boosted by its start of sales in Mongolia, China, the Philippines, and other countries starting in 2022.
K-CAB set new export records in the third and fourth quarters of last year, with exports of KRW 2.5 billion and KRW 3.8 billion, respectively.
K-CAB's cumulative export performance from 2022 is estimated to total at KRW 13.9 billion.
This is the sales volume of K-CAB supplied by HK Inno.N.
Therefore, the company estimates that the sales generated from local prescriptions on-site would be much higher.
K-CAB is considered to be making a smooth start in the overseas market based on its marketability recognized in Korea.
K-CAB's domestic sales last year reached KRW 160.7 billion.
Daewoong Pharmaceutical's Fexuclue recorded KRW 4.7 billion in exports last year.
In 2023, the company recorded its first export value of KRW 400 million, which increased more than tenfold last year.
Fexuclue is the second domestically developed P-CAB class drug introduced, following K-CAB.
Fexuclue is a homegrown new drug that Daewoong Pharmaceutical has successfully developed with its proprietary technology for 13 years since 2008.
Fexuclue has also been launched in Mexico, Ecuador, and Chile.
Fexuclue has entered the market in 30 countries, including South Korea, or is about to enter the market.
The countries that have applied for product licenses are 11 countries, including China, Brazil, and Saudi Arabia.
Daewoong Pharmaceutical has signed export contracts for Fexuclue in 14 countries, including India and the United Arab Emirates.
Fexuclue also made a smooth start in the domestic market.
Fexuclue obtained approval from the Ministry of Food and Drug Safety in December 2021 and began full-scale sales after being listed for reimbursement with the National Health Insurance in July 2022.
Fexuclue posted KRW 97.2 billion in domestic sales last year.
Combined with its export performance, the company posted a total sales of KRW 102 billion, exceeding KRW 100 billion in sales for the first time in 3 years since its launch.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










