- LOGIN
- MemberShip
- 2025-12-21 21:18:18
- Obesity drug craze affects global pharma subsidiaries in KOR
- by Son, Hyung Min | translator Alice Kang | 2025-04-04 05:58:22
Whether or not a new obesity drug in the glucagon-like peptide (GLP-1) family has been launched has had a significant impact on the performance of Novo Nordisk and Lilly Korea.
Last year, Novo Nordisk’s sales increased by 63% upon the launch of Wegovy in the domestic market.
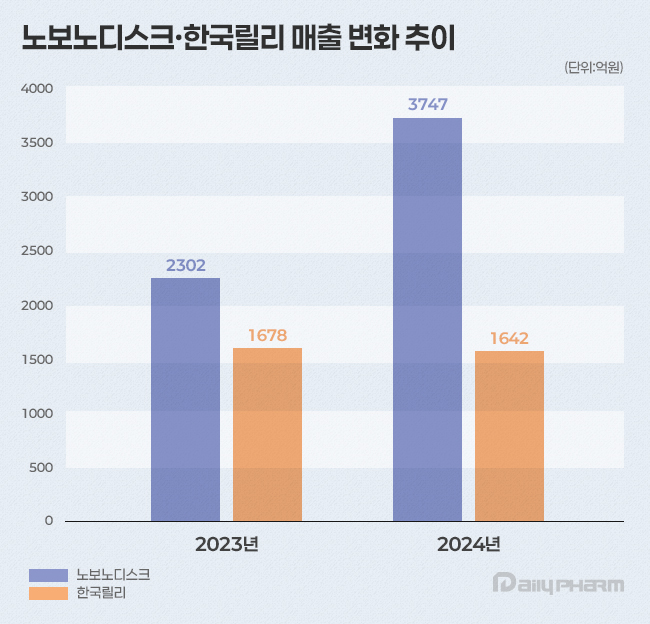
In the case of Lilly Korea, sales decreased slightly due to the delay in the launch of the licensed Mounjaro into the market in 2023 and the sluggish sales of some other products. According to the Financial Supervisory Service on the 3rd, Novo Nordisk Korea's sales increased 63% from KRW 230.2 billion in 2023 to KRW 374.7 billion last year.
Operating profit increased 65% from KRW 8.3 billion to KRW 13.7 billion over the same period.
Novo Nordisk’s sales growth was driven by Wegovy.
According to the market research institution IQVIA, Wegovy recorded sales of KRW 60.3 billion in the same quarter since its launch in October last year.
Wegovy, which was approved in Korea in April 2023, is a GLP-1 formulation composed of semaglutide, which has been confirmed to have effects on reducing weight and glycated hemoglobin.
Novo Nordisk developed the obesity treatment drug Wegovy, a semaglutide that confirmed the weight loss effect of patients during the clinical trial of its GLP-1 class diabetes drug candidate and is administered once a week.
According to the market research institution UBIST, sales of its combination of liraglutide, a GLP-1 analogue, and the insulin degludec, Xultophy, reached KRW 15.1 billion last year, up 26% from the previous year. In addition, the once-weekly insulin product Tresiba and the insulin combination product Ryzodeg also recorded sales of KRW 38 billion and KRW 31.3 billion last year, up 3% and 7%, respectively. Novo Nordisk plans to further strengthen its position in the field of obesity.
Currently, Novo Nordisk is conducting a global Phase III clinical trial of its new obesity drug, ‘CagriSema,' following the success of its previous drugs, Wegovy and Saxenda.
CagriSema is a combination of 2.4 mg of semaglutide, the main ingredient of Wegovy, and 2.4 mg of the long-acting amylin analogue, Cagrilintide.
This drug is regarded as a next-generation obesity drug.
In clinical trials, CagriSema has been shown to be 23% more effective in weight loss than existing single-agent semaglutide.
The expected end of clinical trials is in the first quarter of next year, after which Novo Nordisk plans to apply for approval from major regulatory agencies around the world.
Lilly shows slow performance due to non-launch of Mounjaro On the other hand, the performance of Lilly Korea’s Mounjaro, which is also a GLP-1 class diabetes and obesity drug, showed a slight decline.
Lilly Korea recorded sales of KRW 164.2 billion last year, down 2% from KRW 167.8 billion the previous year.
Operating profit was KRW 10.3 billion, down 1% from 2023.
In the case of Lilly Korea, it is analyzed that sales have not increased significantly due to the delay in the launch of Mounjaro.
Mounjaro is a new diabetes drug developed by Lilly.
Mounjaro acts on both the gastric inhibitory peptide (GIP) receptor and the GLP-1 receptor to promote insulin secretion, improve insulin resistance, and reduce glucagon secretion, thereby reducing blood sugar levels before and after meals.
Mounjaro has proven its weight loss effect through the results of the Phase III SURMOUNT-1 clinical trial, which was conducted on overweight adult patients who are not diabetic, have a body mass index (BMI) of 30 kg/m2 or higher, or have one or more comorbidities, and who were administered Mounjaro once a week.
Lilly launched the same-ingredient obesity treatment, Zepbound, in the US market in November 2023, as it has confirmed the weight loss effect in the clinical trial of the drug in the US.
In Korea, the drug was approved as a treatment for diabetes in June 2023 and secured additional indications as a new obesity drug with the same product name in August last year.
However, its launch in the domestic market has not yet taken place. In addition, sales of Lilly Korea’s Trulicity and Cymbalta were also sluggish last year.
Sales of Trulicity, a GLP-1 class diabetes drug, fell 16% from KRW 44.4 billion the previous year to KRW 37.2 billion.
Sales of Cymbalta, an antidepressant, fell 52% from 2023 to KRW 5.1 billion.
Lilly Korea is looking forward to the success of the SGLT-2 inhibitor Jardiance.
Jardiance's sales last year rose 14% year-on-year to KRW 66.3 billion.
It benefited from the withdrawal of its rival product, Forxiga, from the market last year.
In addition, the company is aiming for a rebound by launching Ebglyss, a new atopic dermatitis drug, in January this year. Lilly is also preparing a next-generation diabetes and obesity drug.
Retatrutide, which is being developed by Lilly, is a next-generation diabetes and obesity drug that acts on three receptors: GLP-1, GIP, and GCG (glucagon).
To date, no new obesity drugs have been commercialized using this mechanism.
In the Phase II clinical trial, Retatrutide demonstrated a weight loss effect of 22.8% and 24.2% when administered at 8 mg and 12 mg at week 48, respectively.
This is a higher weight loss effect than the 20.2% of the existing GLP-1 and GIP targeting Mounjaro.
Currently, Lilly is confirming the potential of retatrutide not only in obesity but also in various chronic diseases such as diabetes and liver disease.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










