- LOGIN
- MemberShip
- 2026-01-02 06:33:29
- OTC drugs 8K↓·health functional foods 25K↑ over 10 years
- by Chon, Seung-Hyun | translator | 2025-12-31 07:49:35
The gap between the number of registered health functional foods and over-the-counter (OTC) drugs in South Korea has widened significantly. Over the past 10 years, health functional foods have more than doubled, and just nine years after overtaking OTC drugs, the number of health functional foods items has reached five times that of OTC products. OTC drugs have decreased to nearly half their previous level over the past 10 years, indicating that the movement to enter the market has been greatly restricted. It was also found that one out of every two approved OTC products has no production record.
According to the '2025 Food and Drug Statistical Annual Publication' released by the Ministry of Food and Drug Safety (MFDS) on the 31st, the number of OTC drug items was calculated at 8,630 last year. This is a decrease of 490 items from 9,120 in 2023.
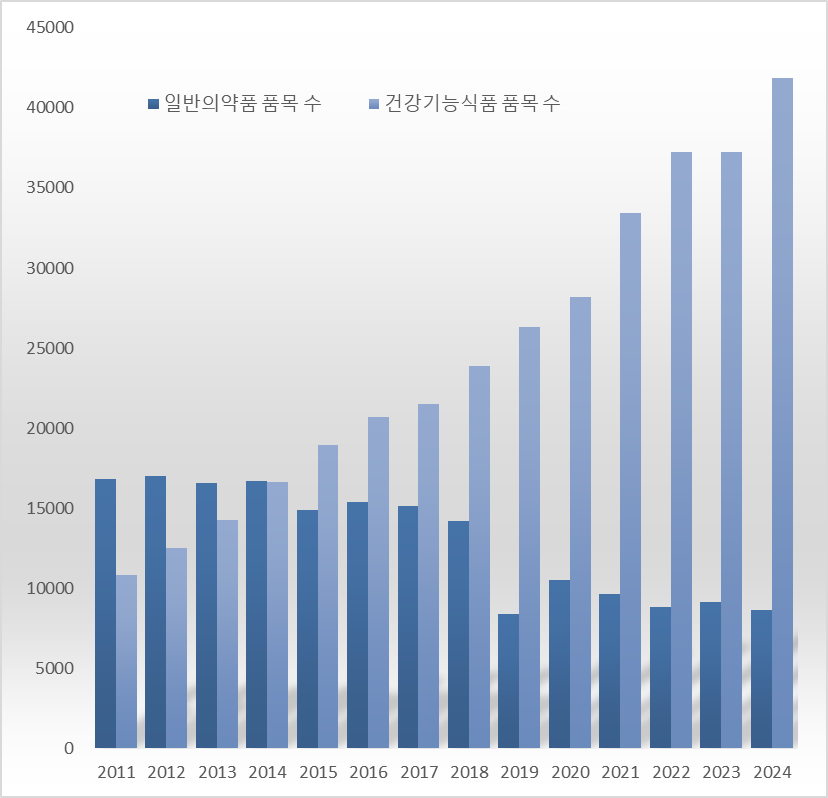
The downward trend in the number of domestically approved OTC drug items is continuing. From 16,717 items in 2014, the count has shrunk to nearly half over the course of 10 years. The number of OTC items dropped by 5,797, from 14,175 in 2018 to 8,378 in 2019, and although it recovered to the 10,000 level in 2020, the decline has persisted since 2021. The number of OTC drug items rose by 307, from 8,813 in 2022 to 9,120 the following year, but returned to a downward trend within a year.
The report indicates that more products have withdrawn from the domestic OTC market than new ones have entered. In the pharmaceutical market, many products disappear due to safety management systems such as the continuous renewal of product licenses. The requirements of the drug product license renewal system is that drugs approved by health authorities must re-verify their efficacy and safety every five years for the license to be maintained. For many products, if marketability is determined to have declined at the time of expiration, the renewal is abandoned and the product is withdrawn from the market.
This is a stark contrast to the continuous annual increase in health functional food.
Last year, the number of manufactured health functional food items was 41,896, an increase of 4,622 from the previous year. Over the 10 years since 2014, when there were 16,632 items, the count has increased by 25,264, expanding by approximately 2.5 times.
In 2014, health functional foods had 85 fewer items than OTC drugs. In 2015, the number of health functional food items rose to 18,956, overtaking the 14,892 OTC drug items by a margin of 4,064, and the gap has gradually widened since then. Last year, the number of health functional food items was found to be about five times greater than that of OTC drugs..
The gap becomes even larger when considering only those OTC drugs with actual production records. Last year, the number of OTC drugs with production records was 4,631, compared to 4,873 in 2023. This means that about half of the OTC drugs currently maintaining MFDS approval have no production records. The number of OTC items with production records has decreased by 1,444 over the 10 years since 2014, when it was 6,075.
Analysis suggests that as the health functional food market size continues to grow and market entry barriers are relatively lower than those for OTC drugs, new entry activities are more active.
Last year, the health functional food market was valued at KRW 4.0131 trillion, a 1.9% decrease from the previous year. It has decreased for two consecutive years from KRW 4.1695 trillion in 2022. The industry analyzes that as new entries into the health functional food market have become active and low-price competition has intensified, it has affected the reduction in market size. However, compared to KRW 2.4130 trillion in 2014, the market size has increased by 146.1%, showing rapid growth recently.
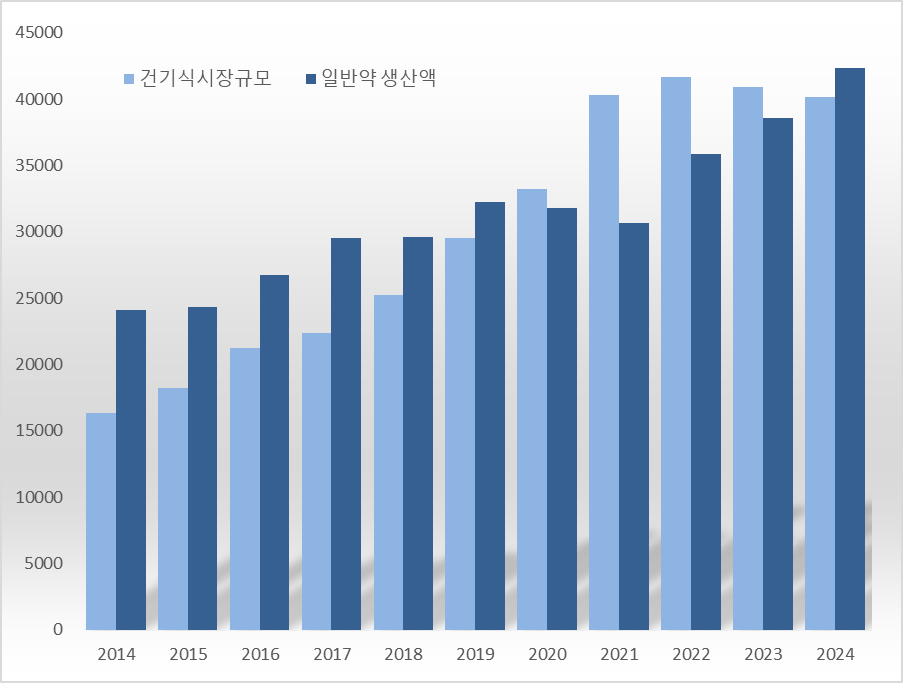
Following the enactment of the Health Functional Food Act in 2003, the health functional food system was implemented. The purpose of introducing the Act was to contribute to the promotion of public health and consumer protection by ensuring the safety and improving the quality of health functional foods and fostering sound distribution and sales. The domestic health functional food market surpassed KRW 1 trillion for the first time in 2010 and exceeded KRW 2 trillion in 2016. The market size grew to the KRW 3 trillion range in 2020 and has remained above KRW 4 trillion since 2021.
While the number of OTC drug items continued to decline, production performance showed a brief upward trend.
Last year, the production size of OTC drugs was KRW 4.2357 trillion, a 9.9% increase from the previous year. Last year's OTC production amount was the largest in history.
The production size of OTC drugs decreased from KRW 3.1779 trillion in 2020 to KRW 3.0692 trillion in 2021 but returned to an upward trend in 2022. In 2022, the OTC production record was KRW 3.5848 trillion, a 16.8% increase from the previous year, and in 2023, it recorded KRW 3.8554 trillion, a 7.5% increase year-on-year. Last year's OTC production scale increased by 38.0% in three years compared to 2021, surpassing KRW 4 trillion for the first time.
Analysis suggests that the recent expansion of OTC production records was most heavily influenced by the COVID-19 pandemic and endemic.
Since the end of 2021, when hundreds of thousands of COVID-19 cases were reported daily, sales of antipyretics, analgesics, and cold medicines used for symptom relief significantly increased. It is analyzed that the boom in the OTC market has continued as the number of flu and cold patients surged since the transition to the COVID-19 endemic phase in 2023.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









