- LOGIN
- MemberShip
- 2025-12-20 14:51:04
- Daiichi Sankyo and Ono Pharmaceutical see surge in sales
- by Son, Hyung Min | translator Alice Kang | 2025-07-14 06:03:52
Japanese pharmaceutical companies operating in South Korea saw overall sales growth last year.
All seven companies surveyed achieved year-on-year sales growth, with Daiichi Sankyo Korea and Ono Pharma Korea leading the way with double-digit growth rates.
However, in terms of operating profits, the companies showed mixed emotions.
According to the Financial Supervisory Service's electronic disclosure system on the 11th, the performance of 7 Japanese pharmaceutical companies with March fiscal year-ends totaled at KRW 1.2499 trillion, up 6.3% from KRW 1.1759 trillion the previous year.
During the same period, operating profit decreased by 11.2% from KRW 92 billion to KRW 81.7 billion.
Major Japanese pharmaceutical companies' Korean subsidiaries follow the Japanese fiscal year, recognizing the period from April last year to March this year as their 2024 annual sales.
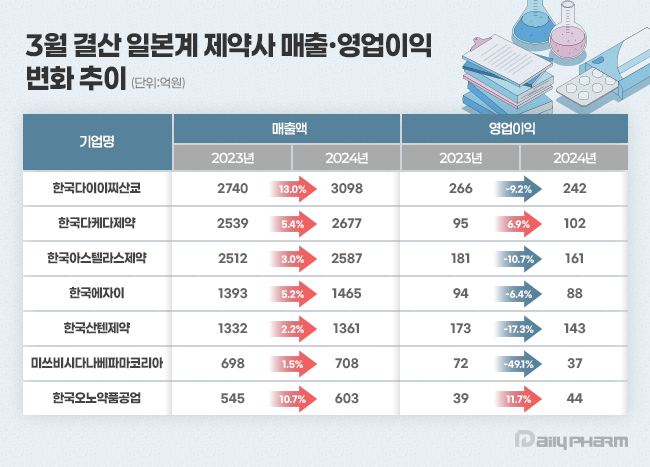
Ono’s sales double in four years Last year, Daiichi Sankyo Korea's sales increased by 13.0% year-on-year to KRW 309.8 billion.
During the same period, operating profit decreased by 9.2% from KRW 26.6 billion to KRW 24.2 billion.
Daiichi Sankyo Korea's sales have been steadily increasing since 2020.
The company surpassed KRW 200 billion in sales for the first time in 2020 with KRW 217.9 billion, followed by KRW 245.4 billion in 2021, KRW 253.2 billion in 2022, and KRW 274 billion in 2023, continuing its upward trend.
In particular, it is analyzed that the company achieved synergistic effects by collaborating with the domestic pharmaceutical company Daewoong Pharmaceutical on certain cardiovascular products such as Lixiana and Sevikar.
Daiichi Sankyo Korea signed a co-marketing agreement with Daewoong Pharmaceutical for Sevikar in 2013 and Lixiana in 2015, and has maintained its partnership with the company to date.
Among these, the highest-selling product is the direct-acting oral anticoagulant (DOAC) Lixiana.
According to market research firm UBIST, Lixiana's prescription sales last year reached KRW 117.5 billion, an 11.6% increase from KRW 105.3 billion in 2023.
The three-component hypertension combination drug Sevikar HCT also maintained its growth momentum.
Last year, Sevikar HCT's prescription sales reached KRW 42.1 billion, up 4.0% from the previous year.
Daiichi Sankyo Korea generated approximately KRW 140 billion in prescription sales from olmesartan-based hypertension treatments, including Sevikar HCT (KRW 42.1 billion), Olmetec (KRW 30.6 billion), and Sevikar (KRW 68.8 billion).
Daiichi Sankyo Korea is working to expand its business from cardiovascular drugs to anticancer drugs.
In particular, the company is focusing on new growth areas such as antibody-drug conjugates (ADCs) by concentrating its R&D capabilities.
Following the approval of Enhertu, the company is preparing to launch various treatments, including five ADC strategies such as Datroway, Patritumab deruxtecan, DS-7300, and DS-6000.
Last year, Takeda Pharmaceutical Korea's sales increased by 5.4% year-on-year to KRW 267.7 billion.
Operating profit rose by 6.9% to KRW 10.2 billion.
Anticancer drugs such as Zejula, Alunbrig, and Ninlaro contributed to the sales growth through steady growth.
According to the market research firm UBIST, Alunbrig recorded prescription sales of KRW 12.1 billion last year.
Since its launch in 2019, its prescription sales have continued to increase.
This treatment is gaining market share for its indication in ALK-positive non-small cell lung cancer.
Alunbrig is in fierce competition with Pfizer's Lorviqua and Roche's Alecensa.
However, However, Alunbrig offers the advantage of once-daily, single-tablet dosing, and can be taken regardless of food intake.
This simplicity reduces the number of factors patients need to consider when taking the medication.
The ovarian cancer treatment Zejula also recorded KRW 14.3 billion in sales last year, a 51.6% increase in prescriptions from the previous year.
In October last year, Zejula was added to the health insurance reimbursement list for the treatment of HRd-positive ovarian cancer.
Until then, Zejula had been reimbursed only as maintenance therapy for BRCA-mutated ovarian cancer patients who responded to platinum-based therapy as a first-line treatment for ovarian cancer.
The growth of Ono Pharma Korea was also noteworthy.
Ono’s sales last year were KRW 60.3 billion, up 10.7% year-on-year.
Operating profit in the same period increased 11.7% from KRW 3.9 billion to KRW 4.4 billion.
The sales and operating profit recorded by Ono last year are the highest figures since the company began submitting audit reports in 2017.
Ono recorded sales of over KRW 40 billion in 2021 and surpassed KRW 50 billion for the first time the following year.
Comparing the KRW 60.3 billion recorded by Ono last year with the KRW 31 billion in sales in 2020, sales increased by 94.5% over four years.
The immune-oncology drug Opdivo drove Ono’s sales growth.
Opdivo is an anti-PD-1 class immuno-oncology drug jointly developed by Ono and BMS, and was approved in Korea in 2015.
Ono holds development and sales rights in Asia, including South Korea, Japan, and Taiwan.
In particular, the combination of Opdivo and BMS's CTLA-4 targeted immunotherapy Yervoy has demonstrated efficacy in various solid tumors such as melanoma, renal cell carcinoma, and hepatocellular carcinoma, contributing to the increase in market share.
According to market research firm IQVIA, Opdivo surpassed KRW 100 billion in domestic sales in 2022.
All showed external growth, but with mixed operating profits Astellas Pharma Korea, Eisai Korea, and Santen Pharmaceutical Korea all saw slight increases in sales, but their operating profits all declined.
Astellas Pharma Korea reported sales of KRW 258.7 billion last year, a 3.0% increase from the previous year.
Harnal, Betmiga, Prograf, and Xtandi performed well, driving sales growth.
Harunal, a treatment for benign prostatic hyperplasia, recorded prescription sales of KRW 66.2 billion last year, a 0.8% increase from the previous year.
Betmiga, a treatment for overactive bladder, and Prograf, an immunosuppressant, have consistently recorded prescription sales of over KRW 30 billion even after their patents expired.
Last year, prescription sales for Betmiga and Prograf were KRW 33.4 billion and KRW 33.6 billion, respectively, up 2.6% and 3.9% year-over-year.
Operating profit decreased by 10.7% year-on-year to KRW 16.1 billion.
A slight increase in the cost of sales contributed to the decrease in gross profit.
Astellas Pharma Korea is pinning its hopes on its next-generation anticancer drugs.
The company's Padcev, its ADC anticancer drug, and Vyloy, its new gastric cancer targeted therapy targeting Claudin 18.2, have entered the domestic market.
Both drugs have shown remarkable results in clinical trials.
Currently, Astellas Pharma Korea has completed its application for insurance reimbursement for both drugs.
Eisai Korea's sales rose 5.2% from KRW 139.3 billion in 2023 to KRW 146.5 billion last year.
Operating profit for the same period decreased 6.4% to KRW 8.8 billion.
Sales of existing products such as the anticancer drug Lenvima, the gastroesophageal reflux disease treatment Pariet, and the Alzheimer's disease treatment Aricept remain steady.
The three products—Aricept (KRW 95.9 billion), Pariet (KRW 17.9 billion), and Lenvima (KRW 12.9 billion)—combined for over KRW 100 billion in sales last year.
Operating profit decreased slightly due to factors such as increased cost of sales.
Lecanemab is an Alzheimer's disease treatment developed by Eisai and Biogen that selectively binds to amyloid beta, a substance believed to cause the disease, and has been proven to slow the progression of the disease and delay cognitive decline.
It is reported that lecanemab is currently being prescribed at various general hospitals and semi-general hospitals in Korea.
Santen Korea reported sales of KRW 136.1 billion last year, a 2.2% increase from the previous year.
However, operating profit decreased by 17.3% to KRW 14.3 billion.
Santen specializes in ophthalmic products and holds various formulations, including diquafosol.
However, in South Korea, hyaluronic acid eye drops are primarily used, and the company has not significantly increased its prescription volume in Korea.
As of last year, the product with the highest sales among Santen Pharmaceutical's portfolio was Cosopt S eye drops, used for glaucoma.
Cosopt S’s prescription volume last year was KRW 33.9 billion, an increase of 2.6% compared to the previous year.
Mitsubishi Tanabe Pharma Korea's sales last year were KRW 70.8 billion, up 1.5% from 2023, but operating profit fell 49.1% in the same period.
The decrease in operating profit was due to an increase in selling, general, and administrative expenses, such as reimbursement and retirement benefits, as well as an increase in cost of sales.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










