- LOGIN
- MemberShip
- 2025-12-24 04:07:00
- GC Biopharma ends its 2-quarter deficit run
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2023-08-02 05:25:41

GC Biopharma explained that the company has succeeded in doing so by increasing sales of highly profitable products and efficient execution of costs.
GC Biopharma also announced plans to accelerate its overseas business with a focus on blood derivatives.
GC Biopharma explained that the company plans to release its immunoglobulin 10% for intravenous administration product within the second half of next year and speed up exports of its blood product plant in Indonesia.
Ends the 2nd quarter streak of operating losses...’Expansion of high-margin product sales + reduced expenditures’ GC Biopharma publicly announced on the 1st that its operating profit in Q2, based on consolidated financial statements, was KRW 23.7 billion, up 80.9% from the KRW 13.1 billion in the same period last year.
Also, sales increased by 2.3% YoY to KRW 432.9 billion.
The company had consecutively recorded an operating loss in Q4 last year and Q1 this year.
The operating loss was KRW 18 billion in Q4 last year and KRW 13.6 billion in Q1 this year.
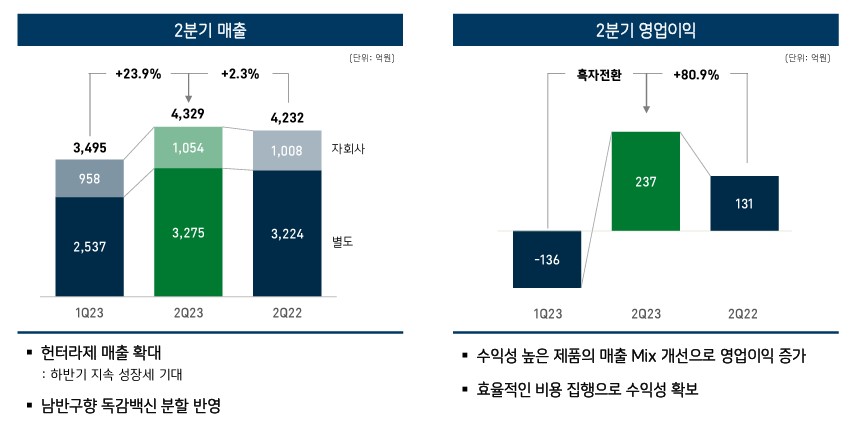
GC Biopharma explained, "We secured profitability by increasing sales of profitable products and efficient cost execution." The company saw the largest increase in its sales of vaccines.
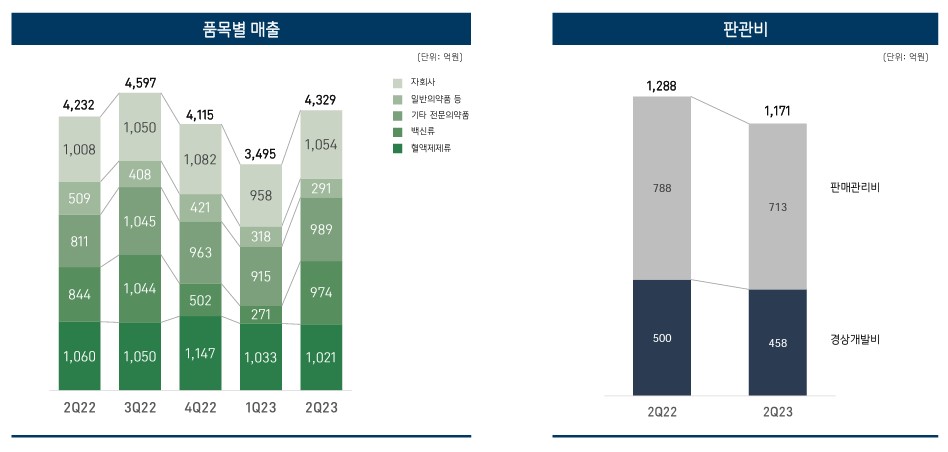
GC biopharma’s vaccine sales had added up to KRW 27.1 billion in Q1 this year, but this had increased 3.6 times to reach KRW 97.4 billion in Q2.
This is a 15.4% increase from the KRW 84.4 billion in Q2 last year.
The analysis is that the separate reflection of sales of flu vaccines exported to the southern hemisphere largely affected the results.
SG&A expenses decreased from KRW 128.8 billion in Q2 of last year to KRW 117.1 billion in Q2 this year.
Among SG&A expenses, ordinary development expenses decreased by 8.4% from KRW 50 billion to KRW 45.8 billion.
within the second half of next year The company also plans to accelerate its overseas business for blood derivative products in the future.
The company’s US project has been attracting the most attention.
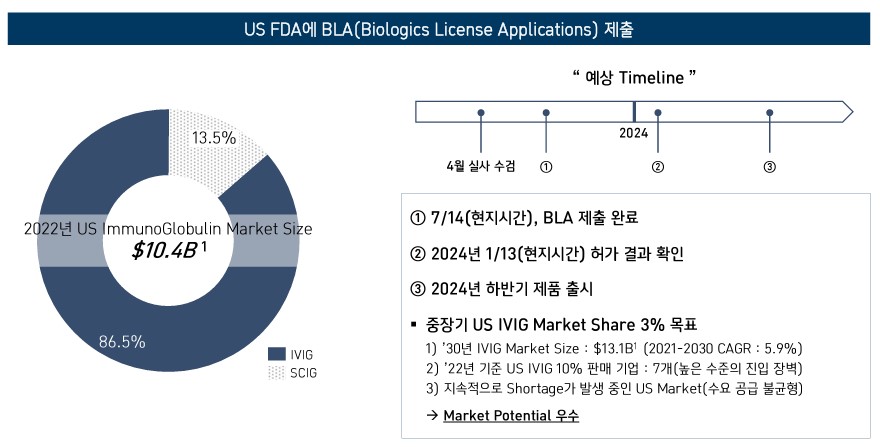
GC Biopharma plans to release an immunoglobulin 10% (IVIG-SN 10%) product for intravenous administration in the US in the second half of next year.
GC Biopharma submitted a BLA to the US Food and Drug Administration (FDA) on the 14th of last month.
FDA’s review results will be confirmed on January 13 next year (local time).
Spurs sales of its blood derivative products...Plans to release ‘IVIG 10%’ in the U.S.
within the second half of next year The company also plans to accelerate its overseas business for blood derivative products in the future.
Among those, the company’s plans in the US have been attracting the most attention.
GC Biopharma plans to release an immunoglobulin 10% (IVIG-SN 10%) product for intravenous administration in the US in the second half of next year.
GC Biopharma submitted a BLA to the US Food and Drug Administration (FDA) on the 14th of last month.
FDA’s review results will be confirmed on January 13 next year (local time).
In the mid-to-long term, the company set the goal of occupying 3% of the IVIG market share in the US.
According to GC Biopharma, the US IVIG market will rise to amount to USD 13.1 billion by 2023 (approximately KRW 16.87 trillion).
As only 7 companies are selling 10% IVIG products in the US, the company’s understanding is that there remains a continuous imbalance between supply and demand in the field.
GC Biopharma was selected as a preferred contractor for blood product plant construction by the Indonesian government in January this year.
The company has also signed business agreements with the Indonesian Red Cross and local pharmaceutical companies.
GC Biopharma expects to be able to execute the main contract within this year.
In Brazil, the company signed a long-term supply agreement for its IVIG 5% product.
Under the agreement it had signed with the Brazilian pharmaceutical company, Blau, GC Biopharma will supply products worth USD 90 million by 2025.
It will additionally supply products until 2028, and the specific supply scale for this will be determined after 2025.
GC Biopharma predicted that profitability would further improve as the price of IVIG products in Brazil is around twice as high as that in Korea.
The company also decided to continue operating its mRNA project.
GC Biopharma plans to complete the mRNA pilot production facility at its Hwasun plant within the year.
For this, the company invested KRW 15 billion in its Hwasun plant in March this year.
GC Biopharma plans to complete the construction of an mRNA pilot production facility this year and begin test operations.

The company, which first has been devloping the treatment as a protein drug, has changed tracks and decided to develop an mRNA drug.
GC Biopharma plans to make the final decision on a candidate substance in the first half of next year.
Since there is currently no product on the market released to treat this disease, GC Biopharma predicts that it will become a first-in-class product if clinical trials are successful.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









