- LOGIN
- MemberShip
- 2025-12-24 00:43:32
- The synergy between technology and sales force
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-08-29 05:28:37
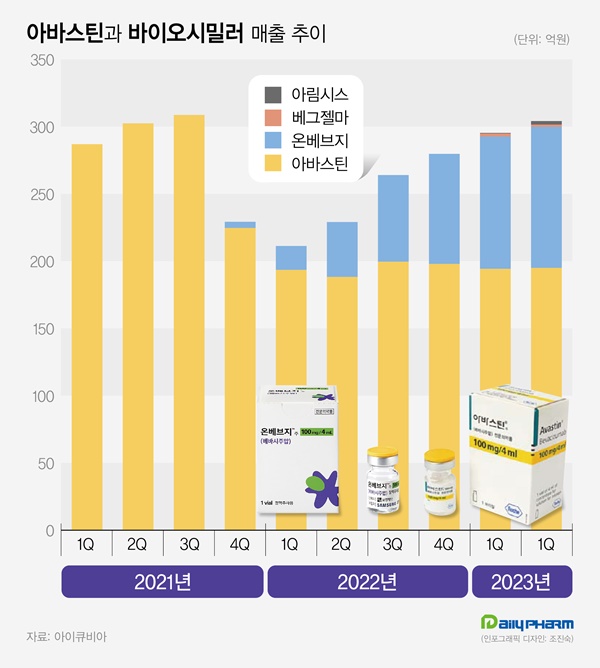
Domestically developed biosimilar products are prominent in the large anticancer drug Asastin market.
Samsungbioepis' Onbevzi exceeded 10 billion won in quarterly sales in the first two years of its release, showing a 35% share.
It is said that Boryung, which was the first biosimilar to enter the market and has differentiated strengths in anticancer drug sales, added to sales and maximized synergy.
According to IQVIA, a pharmaceutical research institute on the 29th, the market size of Bevacizumab in the first half of last year was 59.9 billion won, up 36.1% from the same period last year.
Sales in the first quarter were 29.5 billion won, up 39.8% from the previous year, and continued to grow at 30.4 billion won in the second quarter, a high growth rate of 32.8%.
Bevacizumab is the original medicine of Roche's Avastin.
It is an anticancer drug used for metamorphic direct colorectal cancer and metamorphic breast cancer, non-small cell lung cancer, advanced or metamorphic renal cell carcinoma, glioblastoma, epithelial ovarian cancer, topetic tube cancer, primary peritoneal cancer, and cervical cancer.
Domestically developed biosimilars recently led the expansion of the Bevacizumab market.
In the Avastin market, Samsung Bioepis released the Biosimilar in September 2021, with an additional entry from Celltrion and Alvogen Korea.
Onbevzi's sales in the first half of the year were 20.3 billion won, up 246.9% from the previous year.
Onbevzi's sales in the first quarter were 9.8 billion won, more than five times from 1.8 billion won in the same period last year, and recorded 10.5 billion won in the second quarter, up 157.0% from the previous year.
OnBev is the first time that a biosimilar product released by Samsung Bioepis has exceeded 10 billion won in quarterly sales.
Celltrion and Alvogen Korea, which entered the market this year, have quarterly sales of 100 to 200 million won.
Samsung Bioepis signed a domestic exclusive sales contract with Boryeong shortly after OnBevji's domestic license.
Boryeong is one of the domestic companies that has strengths in the area of anticancer drugs.
Boryung established the anti-cancer division in May 2020.
The organization, which was under the specialty medicine sector, was independent as a separate division.
We have secured the rights to various anticancer drugs and biosimilars owned by domestic and foreign companies, and equipped with gems and notifications as a LBA strategy that buys the rights to the original anticancer drugs.
Boryung also secured domestic rights for Samsung Bioepis' Avastin and Herceptin biosimilar products in 2021.
Boryung is on the rise in the anticancer drug business in the first half of this year alone, with anticancer drug sales of 106.1 billion won, up 48% from the previous year.
From Boryung's point of view, it is also enjoying the effect of improving performance while equipping high-market products.
Samsung Bioepis Samphenet's sales in the first half of the year were 4.1 billion won, up 65.1% from the previous year.
Although the sales volume is not large, the effect of Boryeong's sales force was noticeable.
Sales of the original drug Avastin have not changed much.
In the first half of last year, Asastin's sales were 38.9 billion won, up 2.9% from the previous year.
Asastin's first- and second-quarter sales rose 0.4% and 3.6% year-on-year, respectively.
Avastin showed a stable growth flow of 28.7 billion won, 30.2 billion won and 30.8 billion won from the first quarter to the third quarter of 2021, but it was 22 billion won in the fourth quarter, down 28.6% from the previous quarter.
A decline in sales was inevitable due to the weakening of drug prices following the emergence of biosimilars.
Samsung Bioepis was approved for Avastin's first biosimilar OnBevji in March 2021 and has been listed on the health insurance benefit list since September of the same year.
In October 2021, the upper limit of 0.1g/4mL of Avastin was reduced by 30% from 33,387 won to 231,271 won due to the listing of OnBevji.
Avastin 0.4g/16mL fell 30%.
In principle, when biosimilars appear in the domestic drug price system, the upper limit of original medicines is 30% lower than before the patent expired.
'Innovative pharmaceutical companies, equivalent companies, domestic pharmaceutical companies, and foreign companies that have signed a joint contract, or items that Korea is the first licensed country or products produced in Korea' are guaranteed up to 80% of the original products before the patent expiration of both the original pharmaceuticals and biosimilars.
Because Samsung Bioepis is not an innovative pharmaceutical company, the price of Avastin's drug has fallen to the previous 70% level.
Avastin has experienced a decline in sales of the drug price reduction rate but has since formed similar-scale sales, minimizing the sales gap due to biosimilar penetration.
Onbevji had a 34.5% share of the Bevacizumab market in the second quarter.
Considering that there has been no additional drop in sales since the price drop by Asastin, Onbebge has actually created a new market worth 10 billion won in quarterly sales.
As a result, it is analyzed that the drug price of the original drug also fell by 30% due to the entry of Onbevji, resulting in a significant effect on health insurance finances and the reduction of drug prices for patients.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









