- LOGIN
- MemberShip
- 2025-12-24 00:42:49
- Record number of reimbursed drugs listed in 1 yr
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-10-16 05:24:08
The number of drugs listed for health insurance reimbursement reached a record high in one year.
After the generic market for the diabetes treatment drug ‘Januvia’ opened up, the number of reimbursed drugs expanded rapidly.
With single-agent and combination-drug generics of Januvia that were approved under consignment contracts signed before the enforcement of joint development regulations pouring in, the amount of reimbursed drugs, which had been on a decline after the reform of the drug pricing system, rebounded.
According to the Health Insurance Review and Assessment Service on the 12th, a total of 23,924 drugs were listed for reimbursement as of the 1st of this month.
This is a 291 increase in one month from the 23,633 that were listed until last month.
Last August, the total number of drugs listed for reimbursement was 23,427, which increased by 206 in September, followed by an increase of over 200 this month.
Over the past 2 months, the number of drugs listed for reimbursement increased by 497 in total.
This is the largest market expansion in one year after recording 24,661 in October last year.
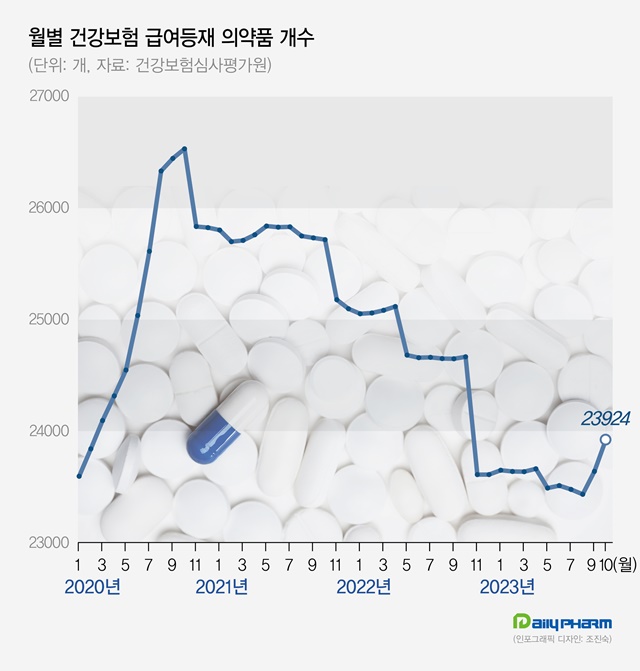
of drugs listed for reimbursement by month Recently, a large number of generic products containing the diabetes treatment ‘sitagliptin’ were listed for reimbursement.
Sitagliptin is the active substance in the DPP-4 inhibitor diabetes drug, 'Januvia'.
According to the Ministry of Health and Welfare, 284 medicines containing sitagliptin were listed for reimbursement on the 1st.
Also, a total of 278 combination drugs that contain sitagliptin and another diabetes drug, metformin, in 7 dosage combinations, were listed.
6 types of sitagliptin and dapagliflozin combination drugs were also listed on the 1st.
Dapagliflozin is the active substance in the diabetes treatment Forxiga.
A large number of sitagliptin generics were listed last month after Januvia’s patent expiry.
On the 2nd of last month, 236 sitagliptin-containing drugs, including 163 single-agent sitagliptin drugs, were listed on Korea’s reimbursement list.
In fact, over the past 2 months, the number of drugs containing sitagliptin has increased significantly in the reimbursement list.
The overall amount of drug coverage, which had been decreasing due to the government's drug price and licensing regulations, has rebounded with the expiration of Januvia's patent.
The number of drugs listed for reimbursement has been on a decline since reaching an all-time high of 26,527 in October 2020.
Last August, the total number of drugs listed for reimbursement was 23,427, which was a decrease of 3,100 in 2 years and 10 months.
This indicates that over the past 2 years and 10 months, 3,100 more drugs were withdrawn or removed from the market than those newly listed for reimbursement under Korea’s health insurance.
The reform of the drug pricing system in 2020 triggered this reduction in the number of new listings.
The main point of the reform enforced in July 2020 was that only generics that satisfy both the ‘direct bioequivalence testing’ and ‘Regulation on Registration of Drug Substances (DMF)’ requirements may receive the highest price among generics at 53.55% of the original drug’s price.
Also, the reform contains a stepped drug pricing system that lowers the price ceiling of generic drugs according to their listing period.
If more than 20 generic drugs are listed in the market for a specific ingredient, the ceiling price for newly listed items thereafter can only be set by up to 85% of the existing lowest price.
As the price of drugs falls drastically under this structure if the generic drug company does not develop the generic drug itself or conduct bioequivalence tests, the approval of generics that are wholly manufactured under consignments has decreased significantly.
The regulatory barriers to licensing have also increased.
With the implementation of the revised Pharmaceutical Affairs Act in July 2021, the number of incrementally modified new drugs and generic drugs that can be approved through a single clinical trial has been restricted.
The new regulation, the so-called '1+3' regulation, contains provisions to limit the number of IMDs and generics that can be approved through a single clinical trial.
If all manufacturing processes are manufactured identically with the same prescription and manufacturing method at the same manufacturing facility as the pharmaceutical company that directly conducted the bioequivalence test, the use of its bioequivalence data is limited to 3 times.
This means that only 4 generic drugs can be approved through 1 bioequivalence test.
Its clinical trial data can also be used for 3 other items in addition to the one by the company that directly carried out the trial.
Before the reform, when a specific pharmaceutical company passed a bioequivalence test and received approval for a generic product, dozens of pharmaceutical companies often received generic approval using the same data.
However, the joint development regulations rendered the ‘unlimited data copying for generic approvals' impossible.
However, unlimited consignment of the recently approved sitagliptin preparation was possible because the contract was made before the implementation of development regulations.
Consignment contracts entered into by pharmaceutical companies before the enforcement of joint development regulations are valid.
In fact, many generics have received approval before the enforcement of joint development regulations, and are waiting to be listed with reimbursement upon expiration of the original drug's patent.
According to the Ministry of Food and Drug Safety, a total of 785 drugs contain sitagliptin.
Of these, 629 have been approved after 2020.
72 and 118 were approved in 2020 and 2021, respectively.
Last year, 254 sitagliptin preparations were approved, and an additional 185 items were approved this year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









