- LOGIN
- MemberShip
- 2025-12-23 22:53:13
- Shingrix lead mkt again…shakes shingles vaccine mkt
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-11-23 05:37:54
The annual KRW 40 billion shingles vaccine market has shown rapid changes.
GlaxoSmithKline's (GSK) new shingles vaccine 'Shingrix' took an unrivaled lead in the Korean market.
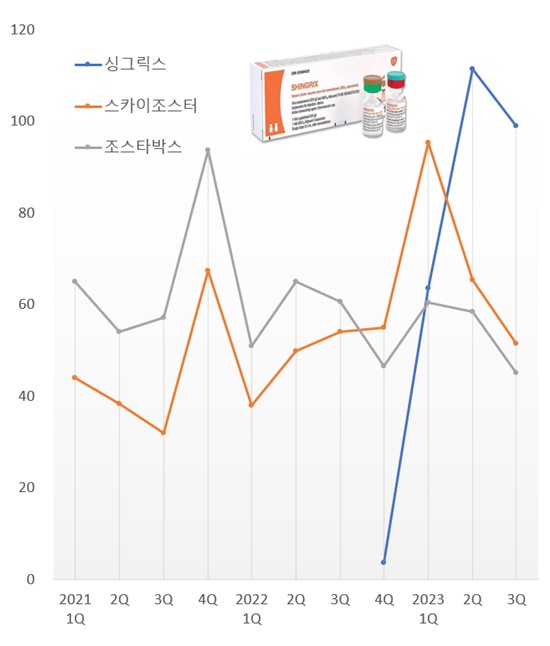
Shingrix’s market share exceeded 50% this quarter powered by its strong preventive effect despite its high price.
According to the market research institution IQVIA on the 22nd, the market for preventive vaccines for shingles in Q3 was KRW 19.5 billion last year, up 70.5% YoY.
The shingles vaccine market, which had posted quarterly sales of around KRW 10 billion last year, had increased greatly this year.
The shingles vaccine market grew 147.0% YoY to record KRW 21.9 billion in Q1 this year, then again rose 124.1% to post KRW 23.5 billion in Q2 this year.
The new vaccine Shingrix drove the market expansion.
Shingrix, which started vaccinations in December last year, showed off its presence by occupying 28.9% of the market with sales of KRW 6 billion in Q1 this year.
In Q2, the drug quickly rose to lead the shingles vaccine market with sales of KRW 11.1 billion.
Shingrix's share of the shingles vaccine market in Q3 reached 50.5% and continued on its unrivaled lead in the market.
In a Phase III clinical trial (ZOE-50) that was conducted on adults aged over 50 years of age, Shingrix showed a 97.2% efficacy compared to the non-vaccinated group at 3.2 years of follow-up, and in another Phase III clinical trial (ZOE-70) conducted on adults aged 70 years and above, Shingrix showed an 89.8% efficacy at 3.7 years of follow up.
This is superior to the 5% protection in adults aged over 50 years of age and 41% in adults aged 70 years and above demonstrated with the use of Zostavax.
The prevention rate of SKYZoster is also known to be similar to Zostavax.
Also, Shingrix’s safety profile was confirmed through 5 clinical trials that were conducted on immunocompromised patients aged 18 years and older.
Based on such evidence, patients who received autologous hematopoietic stem cell transplantation or those with solid cancer, blood cancer, or solid organ transplant patients who have an increased risk of shingles are also eligible to receive vaccination with Shingrix.
At the time of its release, Shingrix's significantly higher price than existing vaccines was pointed to as an obstacle to the vaccine’s early settlement into the market.
vaccines.
The price of Shingrix, which is administered two times in total, is set at around KRW 500,000 to 600,000.
This is more than twice as high as the existing vaccine, which costs KRW 150,000 to 200,000.
However, Shingrix rapidly increased its market share in the market thanks to its superior efficacy despite the high price.
Also, the sales support from 2 domestic pharmaceutical companies contributed to Shingrix’s rapid market penetration.
GSK started domestic sales of Shingrix in partnership with two companies, GC Biopharma and Kwangdong Pharmaceutical.
Existing shingles vaccine products such as SkZzoster and Zostavax saw slower sales growth.
SK Bioscience’s SKYZoster’s sales in Q3 were KRW 5.1 billion, which is a 5.0% decrease YoY.
SKYZoster’s had led the shingles market in Q1 and Q2 this year but then lost the lead to Shingrix.
SKYZoster is a shingles vaccine developed by SK Bioscience with its proprietary technology.
The vaccine has proven its non-inferiority compared to its competitor (Zostavax) in adults aged 50 or older in 8 domestic clinical institutions.
In October 2017, SK Bioscience obtained approval for SKYZoster from the Ministry of Food and Drug Safety for 'preventing shingles in adults over the age of 50'.
Before its approval, MSD's Zostavax was the only vaccine available in the market.
Before then, the market was dominated by MSD's Zostavax, but the introduction of SKYZoster has converted the market into a competitive system.
However, as SKYZoster’s price is less than half of that of Shingrix, there have been estimates that the actual amount of vaccinations made could be larger for SKYZoster than for Shingrix.
Zostavax’s sales in Q3 had fallen 25.6% YoY to record KRW 4.5 billion.
Zostavax’s in Q1 this year rose by 18.9% YoY to reach KRW 6 billion but then fell in Q2 and Q3.
Zostavax gave up its lead to SKYZoster in Q4 last year and then was even surpassed by Shingrix, ranking 3rd in the market.
Zostavax's market share in Q2 had only been 23.0%.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









