- LOGIN
- MemberShip
- 2025-12-23 22:53:14
- Budesonide’s price will be increased by up to 19%
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-11-28 05:41:53
The insurance ceiling price for asthma treatments containing ‘budesonide’ will be increased by up to 18.5%.
The annual prescription market is expected to increase by more than KRW 1.5 billion.
Once the supply and demand imbalance is resolved, the scope of market expansion is expected to increase further.
According to the Ministry of Health and Welfare on the 27th, the prices of Pulmican and Pulmicort will increase starting December 1.
The price of Kuhnil Pharmaceutical's Pulmican will increase by 18.5% from KRW 946 to KRW 1,121.
The price of AstraZeneca's Pulmicort will rise 12.5% from KRW 1,000 to KRW 1,125.
Pulmican and Pulmicort contain budesonide and are used to treat bronchial asthma and acute laryngotracheobronchitis in infants and children.
As supply shortages have frequently occurred due to a recent surge in demand, a decision was made to increase drug prices after discussing with health authorities and pharmaceutical companies to encourage increased production.
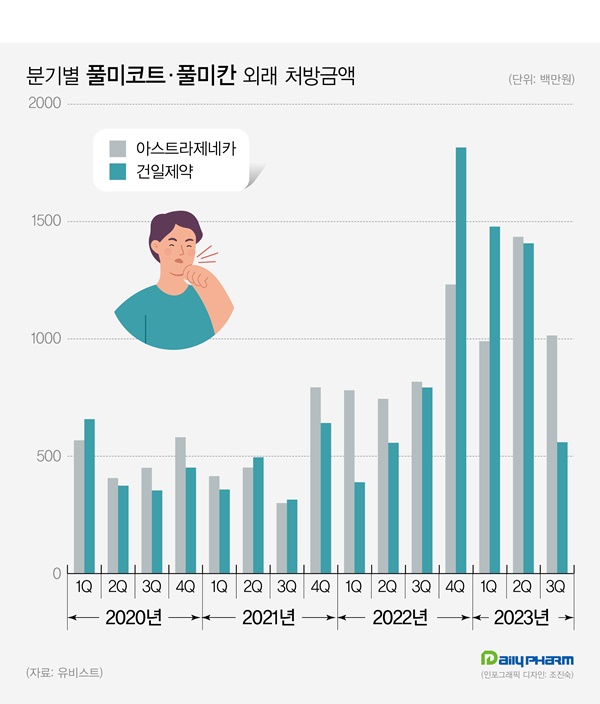
According to the pharmaceutical research institution UBIST, the prescription amount for Pulmicort and Pulmican in Q3 this year was KRW 1.6 billion.
The amount decreased by 0.2% from Q3 last year but is 155.8% in 2 years from the KRW 600 million in Q3 2021.
In 2020, the prescription market for budesonide was only KRW 3.8 billion.
The average quarterly prescription amount was less than KRW 1 billion.
In 2021, prescription performance was less than KRW 1 billion from Q1 to Q3.
Budesonide prescriptions increased 39.0% YoY to KRW 1.4 billion in Q4 2021, then soared to KRW 3 billion in Q4 last year.
This year, sales of budesonide continued to increase, recording KRW 2.5 billion and KRW 2.8 billion in Q1 and Q2, respectively.
The cumulative prescription amount for budesonide in Q3 this year was KRW 6.9 billion, approximately 3 times higher than the KRW 2.3 billion it had raised during the same period in 2021.
The prescription market for budesonide increased significantly with the surge in the number of confirmed COVID-19 cases since the end of 2021.
Recently, the demand for asthma drugs has increased due to the increase in not only confirmed cases of COVID-19 but also cold and flu patients, resulting in a supply-demand imbalance where supply cannot meet demand Prescriptions for Pulmican and Pulmicort both surged from the end of 2021.
Pulmicort’s quarterly prescriptions from 2020 to Q3 2021 ranged around KRW 300 million to 500 million.
It jumped 36.6% YoY to KRW 800 million in Q4 2021 and exceeded KRW 1 billion in Q4 last year.
The cumulative prescription amount for Pulmicort in Q3 this year was KRW 3.4 billion, up 194.7% from the cumulative KRW 1.2 billion in Q3 2021.
Pulmican’s quarterly prescriptions from Q1 2020 to Q3 last year fell below KRW 1 billion.
However, sales surged to KRW 1.8 billion in Q4 last year, and recorded KRW 1.5 billion and KRW 1.4 billion in Q1 and Q2 this year, respectively.
The cumulative prescription amount of Pulmican in Q3 this year was KRW 3.4 billion, a threefold increase from 2 years ago.
The price hike for Pulmicort and Pulmican is expected to expand the prescription market for budesonide.
However, since the market size is not large, its financial burden on Korea’s health insurance is expected to be minimal.
Pulmican recorded prescriptions worth KRW 4.7 billion from Q4 last year to Q3 this year.
If a drug price increase rate of 18.5% is applied, the annual prescription volume will increase by about KRW 900 million.
Pulmicort has recorded prescriptions worth KRW 5.3 billion in the past year, and if its drug price increases by 12.5%, the annual increase is expected to be about KRW 700 million.
When the cost structure of Pulmican and Pulmicort is improved and the supply and demand instability is resolved through increased production, the prescription volume is expected to increase further.
The price hike of Pulmican and Pulmicort is the fourth drug price increase case.
The Ministry of Health and Welfare raised the insurance price ceiling of 18 acetaminophen 650mg items by up to 76.5% in December last year.
The insurance price limit for 650mg acetaminophen, which ranged between KRW 43 to 51 before then, was raised to KRW 90.
The government made an unprecedented decision to raise the price of all acetaminophen together when pharmaceutical companies expressed reluctance to increase production due to the drug’s poor cost structure.
However, it is a temporary increase that will be adjusted to KRW 70 from December this year.
The MOHW has also raised the price of magnesium hydroxide-based laxatives since last June.
The price of Magmil was raised by 27.8% from KRW 18 to KRW 23.
Cho-A Pharmaceutical's Marogel was raised from KRW 15 to KRW 22, and Sinil Pharm’s M Tab Sinil was raised from KRW 16 to KRW 22.
In October, the prices of the 4 types of pseudoephedrine single-agent drugs were increased by up to 45%.
The insurance price of Sinil Pharm’s Pseudoephedrine Tab Sinil increased by 45% from KRW 20 to KRW 29.
The price of Sam Il Pharmaceutical's Sudafed rose 39% from KRW 23 to KRW 32.
The insurance drug price of Sama Pharm’s Schdafen and Kolon Pharmaceutical's Cosue was raised by more than 30% from KRW 23 to KRW 30 and KRW 31, respectively.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









