- LOGIN
- MemberShip
- 2025-12-23 22:53:12
- Domestic market withdrawal of Forxiga remains a mystery
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2023-12-14 05:47:37

This is because the decision to pull out of the Korean market would essentially mean giving up KRW 50 billion in annual sales for AstraZeneca.
While AstraZeneca's official position is that the decision is part of the company’s "portfolio overhaul," the mysteries surrounding the decision are not expected to be resolved anytime soon.
Therefore, the industry's eyes are on AstraZeneca's next move, especially as it has been in stark contrast with MSD’s treatment of Januvia (sitagliptin).
MSD Korea, which had faced a similar situation with Januvia, had sold its domestic sales rights for a large sum to a Korean company.
#SB Company suddenly decides to withdraw ‘Forxiga,’ whose prescription sales exceed KRW 50 billion from the Korean market#EB According to industry sources on the 12th, AstraZeneca Korea decided to withdraw Forxiga from the Korean market.
The withdrawal is limited to the single-agent drug Forxiga.
Its metformin combination Xigduo, sitagliptin combination Sidapvia, and saxagliptin combination Qtern will continue to be supplied through local pharmaceutical companies.
Forxiga is an SGLT-2 inhibitor class diabetes drug.
It was approved in Korea in 2013.
Since then, it has rapidly increased its prescription sales.
The annual prescription amount for the drug exceeded 30 billion won in 2019 and reached the 50 billion won mark last year.
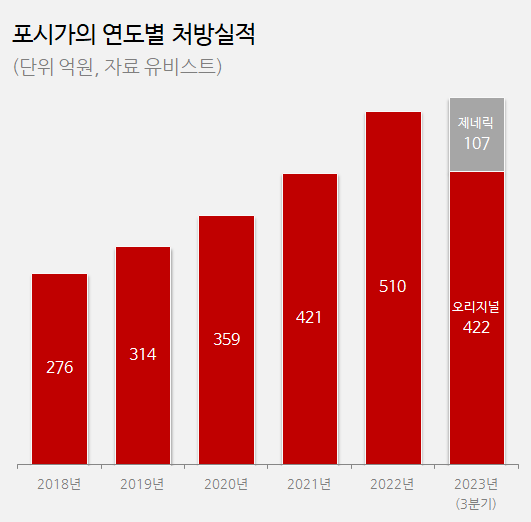
Sales of Astellas' Suglet (ipragliflozin) and MSD's Steglatro (ertugliflozin) accounted for less than KRW 5 billion during the same period.
The joint sales agreement AstraZeneca had made with Daewoong Pharmaceutical is said to have contributed to the increase in prescription performance in Korea.
Daewoong has been jointly selling Forxiga since 2018.
The agreement between the two companies will end at the end of this year.
Forxiga's rise to prominence hit a major inflection point earlier this year.
Forxiga’s substance patent expired in April.
More than 60 pharmaceutical companies launched generic versions of Forxiga upon patent expiry.
This coincided with reimbursement for the use of SGLT-2 and DPP-4 inhibitor combinations, heralding a very fierce competition between the original and generic companies.
AstraZeneca Korea deployed an aggressive defense strategy to maintain Forxiga’s position in the market.
It filed an administrative appeal against the price reduction made upon entry of the generic version.
At the same time, it applied for an injunction for the stay of execution of the government's price reduction.
The court granted the request.
As a result, the price of Forxiga is set to remain at the same level until February next year.
AZ "Drug price reduction disposition unreasonable”…U-turns its stance after half a year The enforcement order was cited on June 1.
However, just over 6 months later, AstraZeneca Korea decided to withdraw Forxiga from the Korean market.
This is a 180-degree turnaround for the company, as this means the company decided to abandon Forxiga just half a year after it challenged the Ministry of Health and Welfare's decision to lower the drug's price with an administrative lawsuit.

In the industry, there are also assumptions that the market withdrawal decision was driven by the rush of generics that entered upon patent expiry and the pressure of excessive competition.
Others argue that AstraZeneca Korea has exhausted resources and accumulated fatigue negotiating with the government to expand reimbursement for its Forxiga and other key products such as Tagrisso.
However, those who believe otherwise point to how Forxiga's influence in the market remains strong even though more than 60 companies have launched generic versions of the drug.
In fact, Forxiga's outpatient prescription performance has increased slightly YoY after the release of its generics.
In Q2, Forxiga's prescriptions totaled to KRW 14.1 billion, up 15% YoY.
In Q3, prescriptions increased 4% YoY to reach KRW 13.7 billion.
While generic products generated KRW 3.9 billion in prescriptions in Q2 and KRW 6.8 billion in Q3, they did so by opening up new markets rather than by taking over Forxiga's share.
Moreover, Forxiga’s indication was being expanded to not only diabetes but also heart failure and kidney disease.
Forxiga has recently added a series of indications.
They were for ▲ the treatment of heart failure with reduced ejection fraction (HFrEF), ▲ left ventricular ejection fraction/ heart failure with preserved ejection fraction (HFpEF)/heart failure with mildly reduced ejection fraction (HFmrEF), and ▲ chronic kidney disease (CKD).
Of these, the company had applied for the drug’s reimbursement in HFrEF and CKD.
The company was also reportedly conducting negotiations with the government for reimbursement extensions until recently.
From AstraZeneca's point of view, extending Forxiga's reimbursement to heart failure could have further increased the prescription amount.
In contrast with MSD's sale of Januvia rights … Competition will intensify to close the KRW 50 billion void The pharma industry focused on how MSD decided to sell Januvia’s domestic sales rights before patent expiry.
Januvia has various similarities to Forxiga, such as the fact that it is a flagship item with a prescription value of KRW 42.6 billion last year and a large number of generics were launched after its patent expiry in July this year.
In May, Chong Kun Dang signed a licensing agreement with MSD to acquire domestic rights for 3 products - Januvia, Janumet, and Janumet XR.
Chong Kun Dang will acquire all domestic sales, distribution, licensing, trademark, and manufacturing rights for the 3 Januvia series drugs.
The contract amount was 45.5 billion won.
Chong Kun Dang paid MSD an upfront payment of KRW 23 billion and additional milestone payments of KRW 22.5 billion based on sales performance.
MSD made a considerable profit by handing over all domestic rights for the antidiabetic drugs to Chong Kun Dang ahead of Januvia's patent expiration, at a time when generics were expected to be launched.
The deal was seen as a win-win, as MSD could focus on sales and marketing of its other products instead of competing with domestic drugmakers, and Chong Kun Dang could secure a stable cash cow that brings in over KRW 100 billion a year.
On the other hand, AstraZeneca made a contrasting move, deciding to withdraw from the Korean market instead of selling the rights for Forxiga to a Korean company.
The company had made no moves to sell Forxiga's domestic rights to any company.
AstraZeneca also drew the line regarding its plans to resupply Forxiga in Korea.
An AstraZeneca Korea official said, "We have secured enough domestic supply to last until the first half of next year.
We are discussing patient protection measures with the Ministry of Food and Drug Safety.
However, at this time, we are not considering resupplying the drug after the first half of next year."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









