- LOGIN
- MemberShip
- 2025-12-23 21:10:34
- Who will take over the Forxiga market worth 50 bil?
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-12-18 05:31:55
The pharmaceutical industry is closely monitoring the potential withdrawal of the diabetes drug Forxiga from the market in Korea.
This withdrawal is expected to intensify competition among companies aiming to fill the market gap left by Forxiga.
Companies that have recently introduced generics of Forxiga are predicted to enter intense market competition.
Furthermore, there is speculation that Daewoong Pharmaceutical, having launched a new medication from the same class this year, could benefit from these developments.
According to the industry, AstraZeneca Korea has officially declared on the 14th its exit from the Korean market with its diabetes treatment Forxiga.
This decision by AstraZeneca to withdraw from the market is attributed to increased competition arising from the introduction of generics, drug price cuts, and restructuring of their portfolio.
Forxiga is a SGLT-2 inhibitor class treatment for diabetes and contains dapagliflozin as its active ingredient.
SGLT-2 inhibitors function by preventing the reabsorption of glucose in the kidneys, which leads to the excretion of glucose via urine and consequently lowers blood sugar levels.
According to the data from UBIST, a market research firm, Forxiga’s prescription totaled to 51 billion won in the previous year.
Therefore, pharmaceutical companies already in the SGLT-2 inhibitor market may likely benefit from the Forxiga's withdrawal.
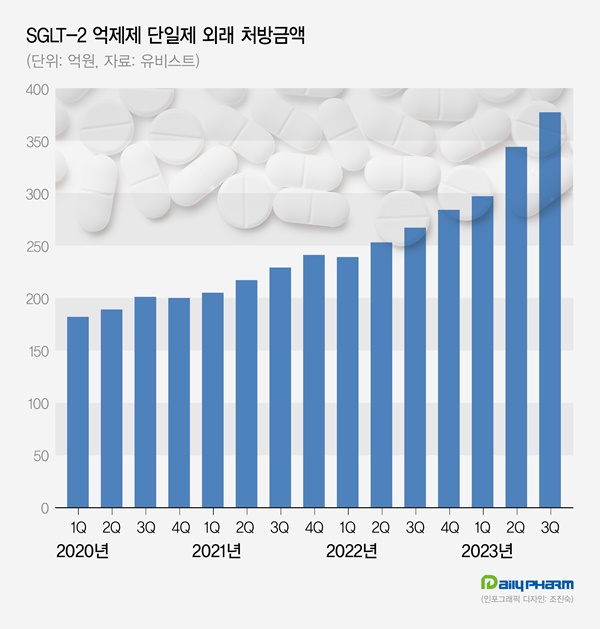
In Q3 of this year, SGLT-2 inhibitors monotherapy achieved outpatient prescription sales of 37.7 billion won.
This reflects a 41.0% increase compared to the previous year.
From Q3 of 2020, where it reached 20.1 billion won in sales, there has been a remarkable 87.2% growth over three years.
SGLT-2 inhibitors, unlike DPP-4 inhibitors which are another class of diabetes treatments, offer an insulin-independent mechanism of action; therefore, they are not affected by insulin resistance.
Additionally, clinical studies that demonstrate benefits in weight loss are seen as a positive factor contributing to the market expansion of SGLT-2 inhibitors.
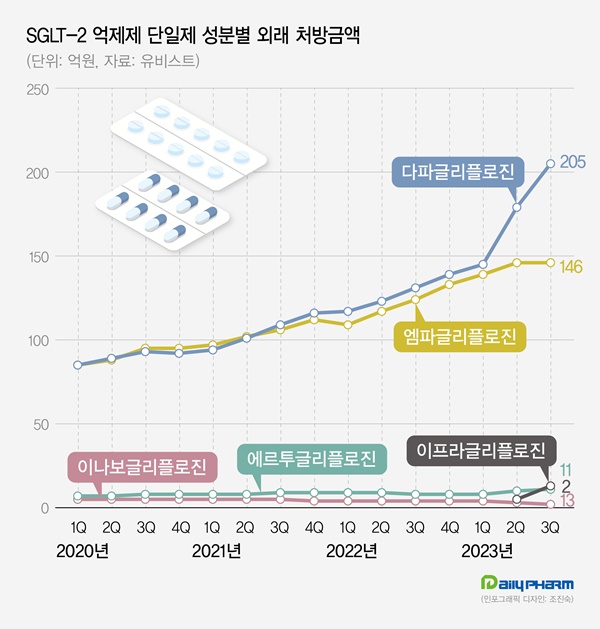
The recent sales for dapagliflozin monotherapy, including Forxiga, have shown a steep increase in growth.
Q3’s prescription sales of dapagliflozin monotherapy reached 20.5 billion won, up 56.0% YoY compared to the last year.
The market size has approximately doubled from the 10.9 billion won in Q3 of 2021, demonstrating substantial expansion over just two years.
The market has significantly expanded recently with the introduction of Forxiga generics.
Following the expiration of Forxiga's substance patent in April, numerous pharmaceutical companies in Korea rushed to release generics containing the dapagliflozin.
Currently, there are approximately 60 companies that have introduced generics of Forxiga monotherapy.
Dapagliflozin’s prescription sales was recorded at 14.5 billion won in Q1, and it increased to 17.9 billion won in Q2, up 23.4%.
Compared to Q1, the prescription sales in Q3 increased by 40.8%.
For pharmaceutical companies, Forxiga's market exit is seen as an opportunity to grow through the substitution of generics that contain the same active ingredient.
In the early stage of the Forxiga generic market, Boryung Pharmaceutical and Hanmi Pharmaceutical are emerging as key players.
Boryung's Trudapa has achieved prescription sales of 1.2 billion won since its introduction, while Hanmi's Daparon has recorded a prescription amount of 1.1 billion won in the past six months.
Additionally, other companies like Chong Kun Dang, Aju Pharm, KyugDong Pharmaceutical, and Daewon Pharmaceutical have also seen prescription figures exceeding 500 million won.
Following Forxiga's withdrawal, prescriptions of other for-2 inhibitors like empagliflozin, ipragliflozin, ertugliflozin, and enavogliflozin may also rise.
Empagliflozin, already a significant player in the SGLT-2 inhibitor market alongside dapagliflozin, is expected to potentially benefit from this market shift.
Empagliflozin monotherapy’s sales from prescription reached 14.6 billion won in the Q3, a YoY 53.0% increase from 9.5 billion won in the Q3 of 2020, demonstrating a strong growth.
As for Empagliflozin-class monotherapy, Boehringer Ingelheim's Jardiance is currently the only product.
Envlo, which contains the active ingredient Enavogliflozin, is Daewoong Pharmaceutical’s SGLT-2 class inhibitor, which was developed for the first time among domestic pharmaceutical companies.
It received domestic approval last year and was launched in May.
Envlo demonstrated superior efficacy with just 0.3 mg, which is less than one-thirtieth of the dose required by existing SGLT-2 inhibitors.
In phase 3 clinical trials involving patients with type 2 diabetes, it proved superior in lowering glycated hemoglobin (HbA1c) and fasting blood sugar levels, as well as in safety, compared to existing drugs.
Since its introduction, Envlo has achieved a prescription sales of 1.6 billion won.
In Q2, it reached 400 million won in prescriptions, which then increased to 1.1 billion won in Q3.
Despite being relatively new to the market and thus having a smaller prescription volume, Envlo has already outperformed the sales performance of ipragliflozin and etugliflozin, making it the third most prescribed drug in its class.
In this sense, Envlo is beginning to establish a significant presence in the market, outshining Forxiga's generics.
Daewoong Pharmaceutical’s past experience in marketing is postulated for the background of Envlo's market expansion.
Since 2018, Daewoong Pharmaceutical has been a co-distributer of Forxiga, and had led its market expansion.
Daewoong Pharmaceutical has proven its sales power in the anti-ulcer drug market.
The company distributed AstraZeneca's PPI class anti-ulcer drug Nexium for 13 years, from 2008 to last year.
This year, they have focused on selling self-developed gastroesophageal reflux disease drug, Fexuclue.
Fexuclue has successfully penetrated the market, recording a prescription amount of 37.4 billion won in its second year until the Q3 of this year.
Last year, Daewoong Pharmaceutical launched Nexierd, containing the active ingredient Esomeprazole, and its cumulative prescription sales in Q3 of this year reached 4.8 billion won, ranking it among the top in its class.
AstraZeneca has secured the domestic supply of Forxiga until the first half of next year and is discussing patient protection measures with the Ministry of Food and Drug Safety (MFDS).
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









