- LOGIN
- MemberShip
- 2025-12-23 21:10:32
- LG Chem joins in the 3-way race in Humira biosimilar mkt
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2023-12-19 05:54:11
LG Chem has joined in to compete for a share of the Humira biosimilar market.
Humira is an original autoimmune disease treatment.
With the introduction, a three-way competition will start between Samsung Bioepis, Celltrion, and LG Chem in the KRW 100 billion annual market.
It has been 2 years since biosimilars entered the Humira market, but the original product still has a stronghold and remains unrivaled in the market.
LG Chem announced on the 15th that it has received marketing authorization from the Ministry of Food and Drug Safety for its adalimumab Humira biosimilar Xelenka.
Two types of Xelenka - Xelenka Prefilled Syringe Inj and Xelenka Autoinjector Inj – were approved at the time.
Xelenka is approved for the following adult indications: rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis, adult Crohn's disease, psoriasis, ulcerative colitis, Behcet's enteritis, hidradenitis suppurativa, and uveitis.
It is also approved for 3 pediatric indications: pediatric Crohn's disease (6 to 17 years of age), pediatric idiopathic arthritis, and pediatric plaque psoriasis.
As a result, three domestic companies, with LG Chem following Samsung Bioepis and Celltrion, have released Humira biosimilars in the market.
In July 2020, Samsung Bioepis received approval for Adalloce, the first Humira biosimilar, followed by the approval of Celltrion’s Yuflyma in June 2021.
Humira posted sales of KRW 104 billion in 2019 and KRW 91.2 billion and 85.8 billion in 2021 and last year, respectively.
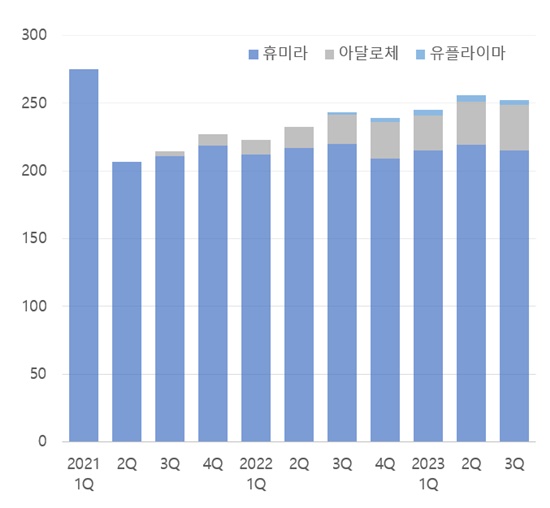
Although 2 years have passed since domestic companies started selling Humira biosimilars, the pace of expansions made by the biosimilars has been deemed to be slow..
According to the market research institution IQVIA, the market for adalimumab reached KRW 25.3 billion in Q3.
This is a 3.7% YoY increase and a 17.6% rise 2 years from the KRW 21.5 billion in Q3 2021.
The original Humira has maintained a solid stronghold over the market despite the entry of its biosimilar.
Humira's Q3 sales decreased 2.3% YoY to KRW 21.5 billion.
This is still a 1.8% rise from the KRW 21.1 billion it had raised in Q3 2021.
Adalloce’s Q3 sales were KRW 3.4 billion.
This was a 54.5% increase from Q3 last year and a 13.3% share of the adalimumab market.
Yuflyma’s Q3 sales reached KRW 400 million.
The market share of the two domestic biosimilars combined was 14.9% in Q3.
The share of biosimilars in the Humira market has been gradually expanding after exceeding 10% in Q4 last year, but the sales gap with the original product remains large.
Experts have attributed the slow market penetration rate to the small gap in the prices of original products and biosimilars.
The insurance ceiling price of Adalloce Prefilled Syringe 40mg/0.4ml and Yuflyma Pen 40mg/0.4ml are KRW 248,877 each, which is only a 15.0% difference from the price of Humira Prefilled Syringe 40mg/0.4ml and Humira Pen 40mg/0.4ml, which are set at KRW 288,091.
In principle, under the Korean drug pricing system, biosimilars can receive insurance prices up to 70% of the original drug price that was set before patent expiry.
Since October 2016, the price of ‘items developed by innovative pharmaceutical companies, or are equivalent, or those developed by domestic pharmaceutical companies in partnership with foreign companies, or items for which Korea grants first approval, or items produced domestically’ are set up to 80% of the original drug’s price.
The price of original drugs whose patents have expired is automatically reduced to 70-80% of its previous level upon the introduction of its biosimilars.
Even if a biosimilar is listed at a price 30% or more lower than the original drug's pre-patent expiry price, as the original drug's price is reduced at the same time, it is difficult for the generic company to secure price competitiveness.
Some analysts believe that in the field of autoimmune diseases, as drugs are used to treat severe illnesses, doctors and patients may prefer new drugs from multinational pharmaceutical companies that have accumulated trust over time if there is not much difference in drug prices.
However, biosimilars are known to contribute to financial savings for national health insurance by lowering the price of original drugs.
As of June 7, 2021, the insurance ceiling price of Humira has been reduced by 30% from the previous price.
The price of 3 drugs, Humira Pen Inj 40mg/0.4mL, Humira Prefilled Syringe Inj 40mg/0.4mL, and Humira Inj 40mg Vial, were cut 30% from KRW 415,058 to KRW 280,891, and the price of Humira Prefilled Syringe Inj 20mg/0.2mL was cut from KRW 224,002 to KRW 156,801.
Humira recorded sales of 27.5 billion won in Q1 2021, but sales had fallen 24.9% the next quarter to 20.7 billion won due to price cuts.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









