- LOGIN
- MemberShip
- 2025-12-23 08:53:24
- Eylea continues to lead AMD mkt despite new competition
- by Son, Hyung-Min | translator Kim, Jung-Ju | 2024-02-26 05:24:45

Eylea continued to top the macular degeneration treatment market last year, posting sales of KRW 96.7 billion.
The Eylea biosimilars Vabysmo and Lucentis, which were released last year, have shown little presence in the market yet.
According to the market research institution IQVIA on Thursday, Eylea’s revenue last year was KRW 96.7 billion, up 20.2% from the KRW 80.4 billion it had made in 2022.
Eylea is a vascular endothelial growth factor-A (VEGF-A) inhibitor for macular degeneration that was developed by Bayer and Regeneron.
The drug was approved in Korea in 2013 and entered the market in earnest the following year after receiving reimbursement approval.
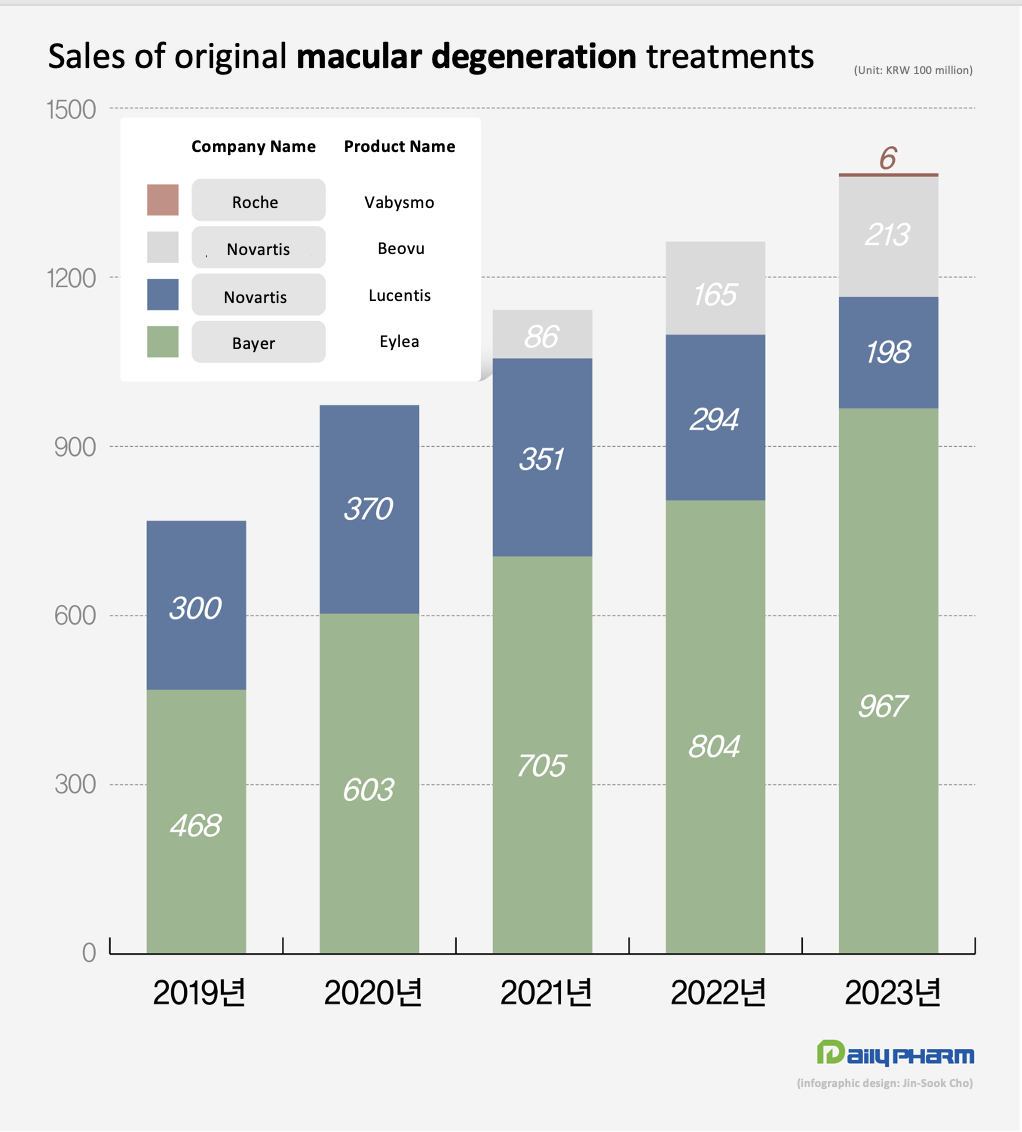
Since then, Eylea’s sales have continued to grow, reaching KRW 46.8 billion in 2019, and then KRW 60.3 billion in 2020.
In 2022, it surpassed the KRW 80 billion mark and reached the KRW 100 billion mark the last year.
However, Eylea now has to fend off an onslaught of biosimilar competitors due to the expiration of its patent last month.
Celltrion, Samsung Bioepis, and Sam Chun Dang Pharm have all made a bid with their Eylea biosimilars.
The original developers, Bayer and Regeneron, are preparing to develop a higher-dose version of Eylea in preparation for the entry of biosimilars.
The companies plan to launch a higher-dose formulation to increase the dosing interval.
The two companies plan to seek approval for the higher-dose Eylea for all of their approved indications, including diabetic macular edema (DME), wet age-related macular degeneration (wAMD), and retinal vein occlusion.
Novartis’s Lucentis posted sales of KRW19.8 billion last year, down 20.2% from the previous year.
Lucentis’s sales have declined steadily since 2020, when it generated KRW 37 billion in sales.
In 2022, the company reported sales of KRW 29.4 billion, and even less, to record sales of less than KRW 20 billion last year.
Lucentis uses the same mechanism of action to inhibit VEGF-A as Eylea but has a shorter dosing interval.
Lucentis must be administered once a month, compared to Eylea, which can be administered once every two months.
Eylea’s efficacy had demonstrated superior vision improvement to Lucentis in patients with severe vision loss due to diabetic macular edema.
Therefore, Novartis plans to focus on marketing its next macular degeneration drug, Beovu.
Like Eylea, Beovu can be dosed once every 2 months.
Beovu, which was released in Korea in Q3 2021, generated sales of KRW 8.6 billion in the same year.
Since then, its sales have continued to grow, generating sales of KRW 16.5 billion in 2022 and KRW 21.3 billion last year.
Total sales of new drugs and biosimilars that entered the market last year amount to KRW 19 billion The Lucentis biosimilars and Roche's Vabysmo, which were newly launched last year, did not make much of an impact in the market the past year.
Chong Kun Dang’s LucenBS, which was the first Lucentis biosimilar to enter the market, sold KRW 500 million, and Amelivu, which is comarketed by Samsung Bioepis and Samil Pharm, sold KRW 800 million last year.
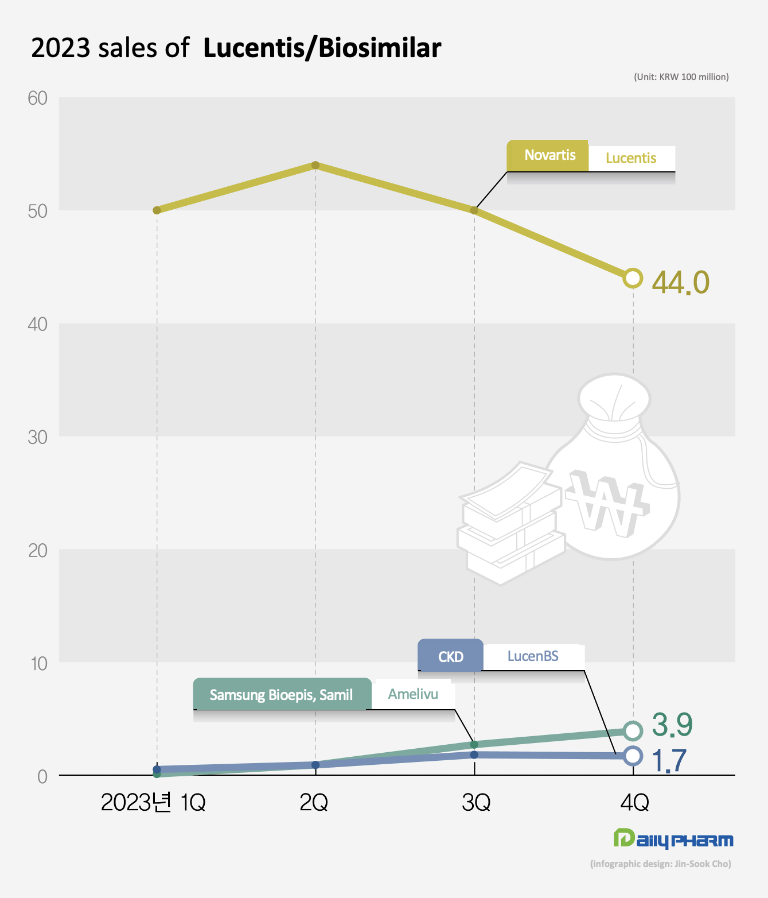
The company cut the price of Lucentis from KRW 300,000 to KRW 150,000 this month.
The original Lucentis costs KRW 580,000 per vial and Samsung Bioepis' Amelivu costs KRW 350,000 per vial.
Because its competitor has significantly reduced the price of its drug, attention is rising to what Samsung Bioepis will do in the next as well.
Vabysmo, which had gained great attention even before its launch, generated KRW 600 million in sales last year.
However, the real competition is expected to start this year, as Vabysmo was granted reimbursement in October last year.
Vabysmo is a macular degeneration treatment that was developed by Roche.
The drug not only inhibits VEGF but also blocks the angiopoietin-2 (Ang-2) pathway to inhibit neovascularization.
Blocking both pathways independently has been shown to be more effective than blocking VEGF alone in reducing inflammation, leakage, and abnormal blood vessel growth.
In particular, in clinical trials, Vabysmo improved visual acuity at a level non-inferior to that of Eylea in the TENAYA and LUCERNE trials that compared its safety and efficacy with Eylea.
Its duration of response lasted 24 months.
In other words, Vabysmo achieved comparable efficacy to other treatments with once every 4 months dosing compared to the once every 1-2 month dosing required for other treatments.
In the global market, Vabysmo’s sales are already closing in on that of Eylea.
Vabysmo generated approximately USD 3.56 trillion in global sales last year, more than half of Eylea’s $7.8 trillion, in just 2 years after its launch.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









