- LOGIN
- MemberShip
- 2025-12-23 09:02:47
- Immuno-oncology market triples in 4 years
- by Son, Hyung-Min | translator Kim, Jung-Ju | 2024-02-28 05:50:15
Sales in the immuno-oncology market surpassed KRW 700 billion last year, driven by the surge in sales of Keytruda and Opdivo.
The market has more than tripled in size over the past 4 years.
Keytruda and Opdivo together accounted for 72.4% of the market and generated more than KRW 500 billion in sales.
The market’s prospects are also bright with major immuno-oncology drugs showing additional efficacy in various solid tumors.
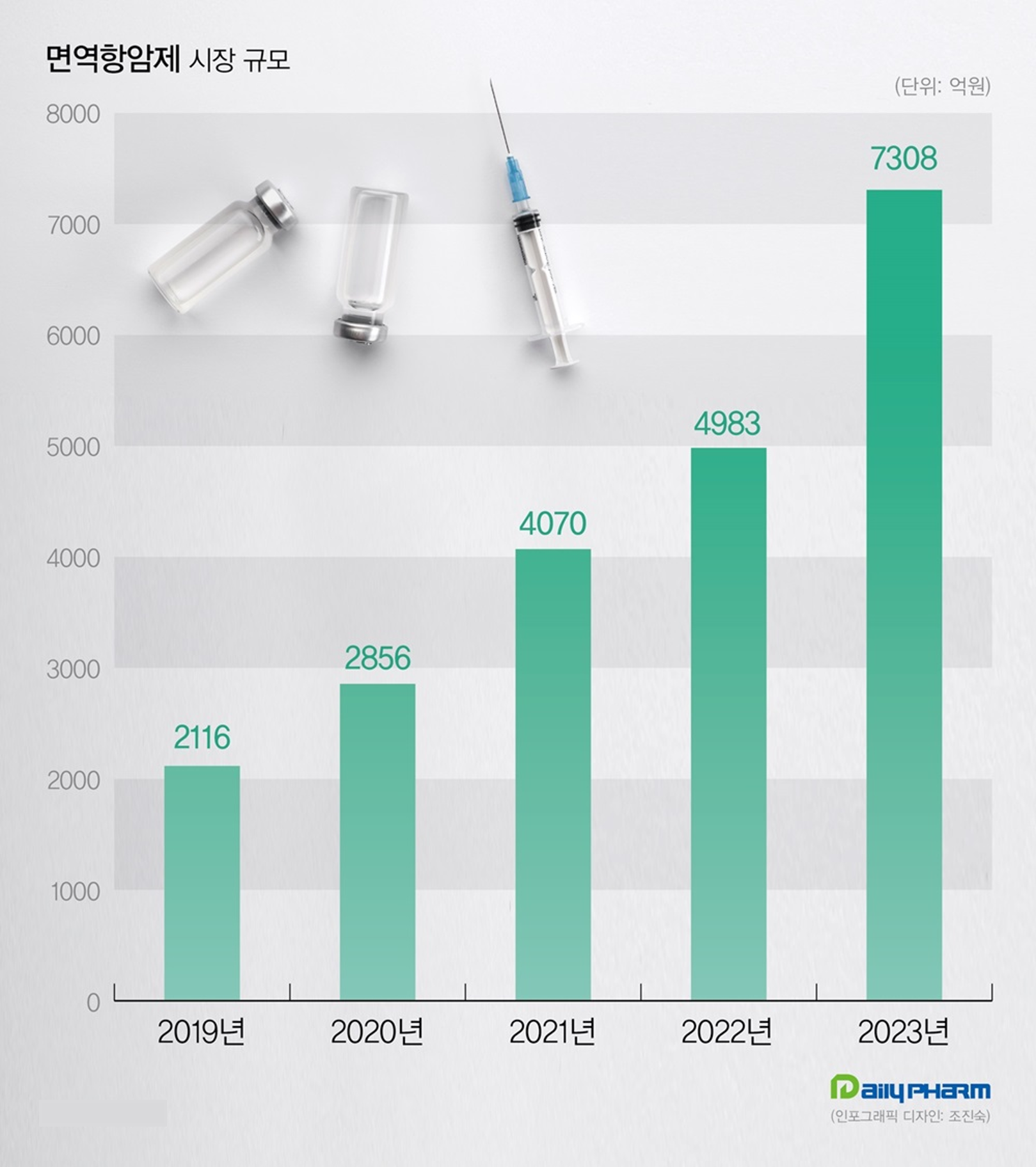
According to the market research institution IQVIA on the 27th, the total immuno-oncology treatment market has recorded KRW 730.8 billion in the past year, a 46.6% increase from the KRW 498.3 billion in the previous year.
Sales have been growing steeply in the immuno-oncology market.
The market, which was worth KRW 200 billion in 2019, reached KRW 700 billion last year after recording KRW 407 billion in 2021 and KRW 498.3 billion in 2022.
This is why the drugs have expanded their indications to several solid tumors, and sales are surging.
In addition to their better effect, immuno-oncology drugs are also known to have fewer side effects than first-generation cytotoxic anti-cancer drugs and second-generation targeted anticancer drugs.
Since the immuno-oncology drugs work by strengthening the body's immune system, side effects such as hair loss, vomiting, nausea, diarrhea, and bone marrow suppression are relatively mild.
Keytruda, which owns 26 indications, approaches KRW 400 billion in sales...Opdivo’s sales surpass the KRW 100 billion mark #EB The market leader is MSD's Keytruda.
Keytruda generated KRW 398.7 billion in sales last year, up 66.4% YoY.
It occupied 54.6% of the market.
Keytruda is approved for 26 approved indications in Korea, owning the most amount of indications among all cancer drugs.
However, only 4 of the cancers are reimbursable: lung cancer, Hodgkin's lymphoma, urothelial carcinoma, and melanoma.
MSD is seeking reimbursement for a variety of other solid tumors, including triple-negative breast and head and neck cancer.
Keytruda's sales may surge further if its reimbursement is further extended.
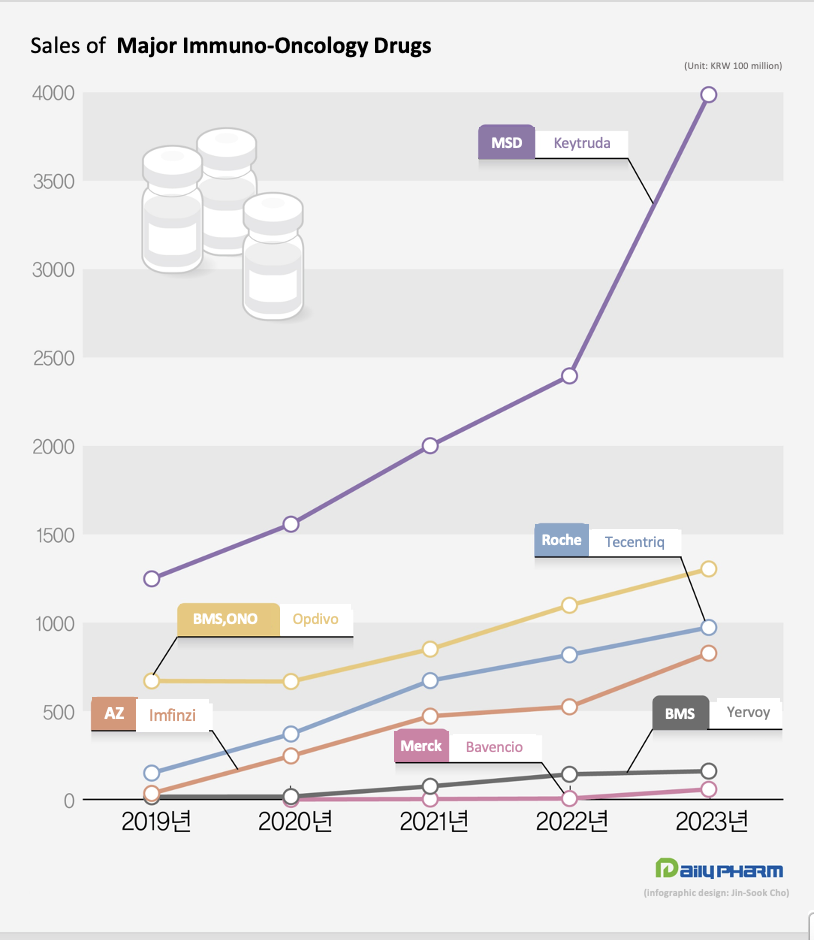
Opdivo generated KRW 130.4 billion in sales last year, up 18.7% from KRW 109.8 billion in 2022.
After hovering in the KRW 60 billion range from 2019 to 2020, the company surpassed the KRW 100 billion mark in 2022 after reaching KRW 85 billion in 2019.
Together, Keytruda and Opdivo generated KRW 529.1 billion in sales last year, accounting for 72.4% of the market.
Opdivo is a PD-1 class immuno-oncology drug co-developed by BMS and Japan's Ono Pharmaceutical.
It has 22 approved indications in Korea, including melanoma, non-small cell lung cancer, and renal cell carcinoma.
Both companies have been steadily expanding the number of their solid tumor indication since their approval.
In addition to extending Opdivo’s reimbursement coverage, BMS and Ono plan to develop a subcutaneous (SC) formulation to expand options for the patients.
Opdivo's major patents are set to expire in 2028.
BMS confirmed that Opdivo’s subcutaneous (SC) formulation was non-inferior to the intravenous (IV) formulation in a Phase III clinical trial conducted to confirm the efficacy of the SC formulation.
The safety results were also comparable in both arms.
The SC formulation can be administered in less than 5 minutes, compared to the 1 hour required for the existing IV formulation.
BMS is preparing to apply for approval of its SC formulation with regulatory authorities around the world.
Tecentriq and Imfinzi gains ground in intractable cancers Roche's Tecentriq and AstraZeneca's Imfinzi also showed marked sales growth.
After posting KRW 37 billion in 2020 and doubling its sales in 2021, Tecentriq’s sales have been on a steady rise.
Roche has been rapidly expanding Tecentriq’s indication by actively accepting the government’s requests.
Tysentriq gained coverage for lung cancer within a year of approval and expanded coverage in 2019 by removing the PD-L1 expression limit.
Roche plans to expand Tecentriq’s indications through combined use with targeted cancer drugs.
Tecentriq, in combination with Avastin, became the first immuno-oncology drug to be reimbursed for the first-line treatment of liver cancer.
Roche is also conducting combination trials with NT-I7, an anti-interleukin (IL)-7 inhibitor developed by a Korean biotech NeoImmuneTech, in lung and skin cancers.
AstraZeneca's Imfinzi posted sales of KRW 82.7 billion last year, up 57.8% from KRW 52.4 billion in 2022.
After generating KRW 3.4 billion in sales in 2019, Imfinzi’s sales surged 624% to KRW 24.6 billion the following year.
With sales exceeding KRW 80 billion last year, Imfinzi ranked fourth in the immuno-oncology market.
Imfinzi’s strength is that it is the first immuno-oncology drug to show efficacy in biliary tract cancer.
In 2022, AstraZeneca received approval for the Imfinzi+gemcitabine+cisplatin combination in Korea.
This approval established the Imfinzi combination as the new standard of care in biliary tract cancer for 12 years.
AstraZeneca is currently in the process of extending Imfinzi’s reimbursement to its biliary tract cancer indication.
BMS plans to extend indications for the Yervoy+Opdivo combo...Bavencio’s sales increase with reimbursement in urothelial cancer
Sales of Yervoy, which is the only immuno-oncology drug to target CTLA-4, grew only 12.6% last year, generating sales of KRW 16 billion.
In 2021, Yervoy received approval for use in combination with Opdivo in 2021.
However, the maximum dosing interval was set at 2 years, meaning that even if it works, patients will not be eligible for additional reimbursement afterward.
BMS plans to explore multiple uses for the Yervoy and Opdivo combination.
The company is currently exploring the combination of metastatic colorectal cancer, squamous cell carcinoma, and head and neck cancer.
Merck's Bavencio generated KRW 5.7 billion in sales last year.
Sales of Bavencio grew with its reimbursement approval in metastatic urothelial cancer.
Bavencio, which was approved in Korea in 2019, secured reimbursement as a first-line maintenance therapy for urothelial cancer in 2023.
The variable is Padcev.
Padcev, which is an antibody-drug conjugate (ADC) cancer drug developed by Astellas and Seegen, has confirmed efficacy results as a first-line treatment in urothelial cancer.
Based on the results of the study, the company is accelerating its efforts to receive approval as a first-line treatment in urothelial cancer This could have implications for Bavencio, as it is being used as a maintenance therapy in the first-line setting.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









