- LOGIN
- MemberShip
- 2025-12-23 08:53:25
- Sales of Tagrisso stay strong, Leclaza rises in lung cancer
- by | translator Kim, Jung-Ju | 2024-03-20 05:44:08
Tagrisso and Leclaza are both third-generation targeted therapies, and the two were approved as a first-line treatment for lung cancer this year and were given the green light to expand sales.
Among first- and second-generation targeted therapies, all drugs other than Giotrif posted sluggish sales last year.
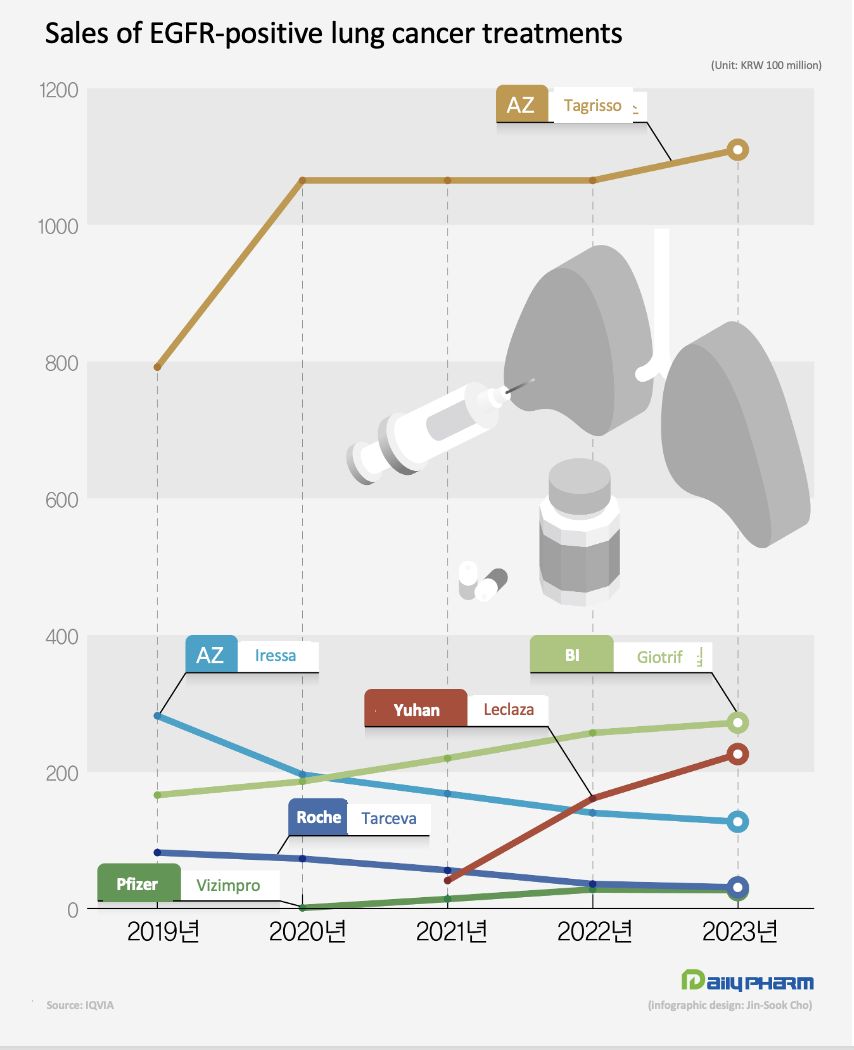
According to the market research institution IQVIA on the 20th, Tagrisso generated sales of KRW 111 billion last year, up 4.2% YoY.
Tagrisso is a third-generation tyrosine kinase inhibitor (TKI) that was developed by AstraZeneca.
EGFR-positive lung cancer treatments are categorized into four types: first-generation EGFR TKIs like AstraZeneca's Iressa (gefitinib) and Roche's Tarceva (erlotinib); second-generation EGFR TKIs like Boheringer Ingelheim's Giotrif and Pfizer's Vizimpro (dacomitinib); and third-generation EGFR TKIs like Leclaza (lasertinib) and AstraZeneca's Tagrisso (osimertinib).
Sales of Tagrisso, which exceeded the KRW 100 billion mark in sales in 2020, have remained stagnant for 2 years.
After reaching KRW 106.5 billion in 2020, the drug posted similar sales in 2021 and 2022.
After working to receive reimbursement for the drug as a first-line treatment for years, AstraZeneca received the reimbursement approval this year as a first-line treatment for EGFR-positive lung cancer, and this is also expected to expand sales.
Moreover, Tagrisso is also the only third-generation TKI to be approved for use in early-stage lung cancer.
In December last year, Tagrisso was approved as an adjuvant therapy for patients with EGFR exon 19 and exon 21 mutated NSCLC after complete resection.
In the phase III ADAURA study, Tagrisso was shown to reduce the risk of death by 51% compared to conventional therapy.
Powered by the advantages, Tagrisso’s sales are expected to increase further in the coming years.
Leclaza posted sales of KRW 22.6 billion last year, up 40.3% YoY.
Leclaza, which was approved in Korea in January 2021, was also granted reimbursement the same year.
After posting KRW 4.1 billion in sales in Q2, it posted sales of KRW 16 billion the following year, then surpassed KRW 20 billion last year.
Before the approval, patients had to be confirmed to be T790M-positive through an additional tissue test after using a first- or second-generation EGFR TKI to be eligible to use Leclaza with reimbursement.
With all 3 TKIs, including Leclaza and Tagrisso, now covered as first-line treatments, physicians and patients will have a full range of first- and third-generation targeted therapies to choose from.
Another benefit of Leclaza is its potential for use in combination with Rybrevant.
Combination therapies, including Tagrisso plus platinum-based chemotherapy and Rybrevant plus platinum-based chemotherapy, are currently approved by regulatory authorities abroad for the first-line treatment of lung cancer.
Combining the use of Leclaza, which targets EGFR-mutated exon 19 and exon 21 deletion, with Rybrevant, which targets exon 20 deletion, has been attracting attention as a viable targeted therapy combination.
Currently, the Leclaza + Rybrevant combination is being reviewed for approval in the US as a first-line treatment for lung cancer.
Sales of all first-generation EGFR TKIS other than Giotrif decline During the same period, first- and second-generation TKIs saw sluggish growth.
Only Giotrif’s sales showed growth, while all others declined.
Giotrif generated KRW 27.2 billion in sales last year, up 5.8% from 2022.
Since its launch in Korea in 2014, Giotrif has seen a gradual but steady increase in sales.
After surpassing the KRW 10 billion mark in sales for the first time in 2017, Giotrif posted sales of KRW 16.6 billion in 2019, KRW 22 billion in 2021, and KRW 25.7 billion in 2022.
Giotrif benefited how third-generation TKIs not being used as first-line treatments.
On the other hand, Vizimpro’s sales had been sluggish.
Vizimpro, which had been released later in 2020, had increased slightly to KRW 1.4 billion in 2021 and KRW 2.8 billion in 2022, but stagnated at KRW 2.7 billion last year.
The weakness of Vizympro is in its side effects.
In the ARCHER 1050 study, the Phase III trial that became the grounds for its approval, Vizympro showed better efficacy compared to first-generation TKIs, but also a higher rate of adverse events.
60% of patients in the Vizympro arm required dose adjustments due to adverse events.
In general, first-generation TKIs are continuing to show declining sales.
First, Iressa posted sales of KRW 12.7 billion last year, down 9.2% YoY.
Sales have been on a steady decline since 2020 when sales fell below KRW 20 billion (USD 19.6 billion).
Iressa posted KRW 16.7 billion in sales in 2021 and KRW 14 billion in 2022.
Iressa is known to be a milder cancer drug with fewer side effects among targeted therapy options.
As such, it has been used as a priority in patients with relatively poor health.
However, sales have been declining with more treatment options becoming available, with latecomers confirming their efficacy over Iressa.
Tarceva’s sales fell 13.8% YoY to KRW 3.1 billion last year.
This is the fifth consecutive year the drug saw declining sales after posting KRW 8.2 billion in 2019.
Compared to the KRW 17.3 billion it had earned in 2016, Tarceva’s sales have more than halved.
Tarceva’s sales are also on a decline due to the emergence of more effective second and third-generation targeted therapies.
Tarceva also suffered a setback in 2016 when its maintenance therapy indication was removed for patients with stage IIIA or higher NSCLC.
Also, Tarceva was not proven effective in retrospective clinical studies.
Therefore, its sales are expected to continue to decline when combined targeted therapies are approved for use in the first line.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









