- LOGIN
- MemberShip
- 2025-12-22 20:45:12
- Company
- 'Xtandi and Erleada' compete in the prostate cancer market
- by Hwang, Byung-woo Aug 14, 2024 05:51am
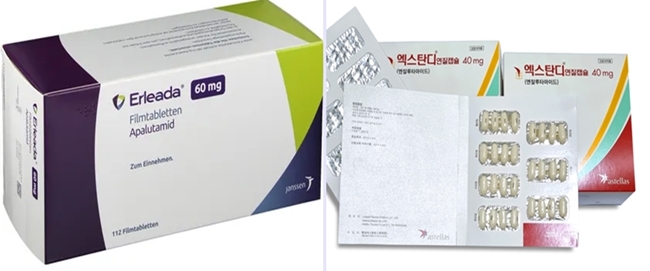
- The market for ARTA treatment used to treat prostate cancer has been dominated by Xtandi (enzalutamide). As prescription sales of Erleada (apalutamide) rise, the market is shifting. Astellas Pharma's Xtandi has increased its competitiveness with its indications, Janssen has started to face competition by expanding its portfolio, including Erleada, Zytiga, and Akeega. (From left) product photos of Erleada and Xtandi According to the pharmaceutical market research firm UBIST, Xtandi generated the highest outpatient prescription sales in this year's first half, KRW 14.1 billion. Analysis suggests that increased outpatient prescription sales are due to expanded reimbursement. In August 2022, Xtandi was selectively reimbursed for the treatment of patients with advanced prostate cancer accompanied by distant metastasis when used in combination with androgen deprivation therapy (ADT). Since November of last year, reimbursement has been made regardless of using other androgen synthesis inhibitors. However, the drug price was reduced from KRW 20,882 to KRW 14,170 due to the expanded reimbursement range, affecting prescription sales. Xtandi's prescription sales for this year's Q3 was KRW 8.1 billion, then reduced to KRW 6.6 billion in Q4. In November last year, the co-payment rate for Xtandi was adjusted from 30% to 5%, canceling out the prescription price reduction. Xtandi's sales recovered, with KRW 6.7 billion in this year's Q1 and KRW 7.4 billion in Q2. Also, in June, Xtandi expanded its indication to non-metastatic hormone-sensitive prostate cancer (nmHSPC), which is expected to increase prescription sales in the future. Sales trend of ARTA treatments used to treat prostate cancer (UBIST report reconstructed by Daily Pharm). With the expanded indications, Xtandi became the only ARTA treatment that can be prescribed to treat all prostate cancer stages following biochemical recurrence, including hormone-sensitive, castration-resistant, non-metastatic, and metastatic. An Astellas official said, "Xtandi is the only prostate cancer treatment that can be used to treat hormone-sensitive HSPC at early stages regardless of metastasis, non-metastasis, and the number of metastases." He said, "When the indication to treat nmHSPC was recently approved, the company focused on marketing to raise awareness of the importance of early ARTA combination therapy to treat HSPC." Erleada chasing Xtandi…Janssen, "To provide patient-individualized treatment option" Although Xtandi is the market leader, Janssen's Erleada, which has been reimbursed since last year, rapidly generates prescription sales. Erleada generated prescription sales of KRW 3.4 billion in last year's Q4. Its sales grew with a bigger margin, with KRW 4.8 billion in this year's Q1 and KRW 7 billion in Q2. Its prescription sales in Q2 were close to Xtandi. Analysis suggests that the close competition may be due to Xtandi's drug price reduction. Erleada appears to be established in clinical practice, as it has been a year since receiving reimbursement. On the other hand, the prescription sales of Janssen's Zytiga (abiraterone) have decreased after the introduction of generic versions. Last year, prescription sales for Xytiga decreased from KRW 4.9 billion in Q3 to KRW 3.5 billion in Q4. In this year's Q1 and Q2, it generated KRW 3.1 billion due to expanded prescriptions of competitor drugs and the introduction of generic products. After Hanmi Pharm's Abiterone was approved last year, generic version of Xytiga were introduced to the market for Xytiga. Furthermore, Hanmi Pharm launched the combination drug Abiterone Duo in February, and Ace Pharmaceuticals obtained approval for Amaron. With more competitors, Xytiga's prescription sales are expected to decrease even more. Changes to the reimbursed price of ARTA treatments used to treat prostate cancer (HIRA report reconstructed by Daily Pharm). Janssen plans to focus on expanding treatment options for prostate cancer sub-types to compete against Xtandi, which has broad indications. Janssen's prostate cancer treatment portfolio consists of Xytiga, Erleada, and Akeega. Akeega was approved in September of last year. Akeega is a treatment for BRCA1/2 mutation-positive metastatic castration-resistant prostate cancer. It is a combination drug combining abiraterone and the PARP inhibitor ingredient niraparib. Prescription sales of individual drugs may be lower than those of Xtandi, but the company's strategy is to face the competition by diversifying treatment options. A Janssen official said, "For the treatment of prostate cancer in South Korea, we have been putting efforts to provide various individualized treatment options and improve treatment accessibility." "We will strive to work as a market leader to provide Korean patients with prostate cancer with optimized treatment by expanding treatment options through Erleada and Xytiga and expanding our portfolio, including Akeega.
- Company
- Nabota accounts for 24% of Daewoong’s ETC sales
- by Chon, Seung-Hyun Aug 13, 2024 05:47am
- Daewoong Pharmaceutical's botulinum toxin 'Nabota' continued its upward sales trend. The company's quarterly revenue exceeded KRW 50 billion for the first time, led by the company’s growth in overseas markets. Nabota’s share of Daewoong's specialty drug sales reached nearly 25 percent, driving the company's performance. According to Daewoong Pharmaceutical on the 12th, Nabota generated sales of KRW 53.1 billion in Q2, up 62.4% year-on-year. This is the first time since its launch that the drug’s quarterly sales exceeded KRW 50 billion, surpassing the previous record of KRW 42.6 billion set in Q1 last year in less than a year. Nabota’s sales increased 74.7% in 2 years, compared to sales of KRW 30.4 billion in Q1 2022. Daewoong Pharmaceutical Sales of Nabota in the U.S. market continued to rise steadily. Daewoong Pharmaceutical's sales partner for Nabota, Evolus, reported sales of USD 66.9 million (approximately KRW 92 billion) in Q2, up 35.6% from the USD 49.35 million made year-on-year. This marks the fifth consecutive quarter Evolus renewed its revenue record since the company reported USD 49 million in Q2 last year. Nabota's export performance has begun to surge since the closure of its strain theft lawsuit with Medytox, which began in 2019, and the established trust in Nabota based on the accumulated U.S. experience. In February 2021, Medytox entered into a settlement agreement with Daewoong Pharmaceutical's U.S. partners Evolus and AbbVie for the sale of Nabota (U.S. trade name Jeuveau) in the United States. Under the agreement, Medytox and AbbVie received the rights to continue marketing and distributing Jeuveau in the U.S. for certain payments from Evolus. Evolus is also looking to expand into the European market, having launched Nabota in Spain in June. In Europe, Nabota is currently available under the brand name Nuceiva and is now available in the U.K., Germany, Austria, Italy, and the U.S., in addition to Spain. In June, the company received marketing authorization for Nabota from Argentina's National Administration of Drugs, Food and Medical Devices (ANMAT), accelerating its international expansion as well. Nabota will be launched in Argentina under the brand name Clodew in Q4 this year through Daewoong’s local partner Oxapharma. Daewoong Pharmaceutical currently has botulinum toxin products licensed in more than 70 countries worldwide and partnerships in more than 80 countries. As a result, Nabota's presence in Daewoong's sales is also gradually expanding. In Q2 last year, Nabota’s sales accounted for 24.4% of Daewoong's KRW 218 billion in specialty drug sales, the largest share ever. Among the products developed and sold by Daewoong Pharmaceutical, Nabota has generated the largest sales volume. Daewoong's sales of specialty drugs increased 11.7% in 3 years from KRW 181 billion in Q2 2021, while Nabota’s sales more than tripled in the same period. Nabota's share of the company's specialty drug sales has nearly tripled in 3 years from 8.5% in Q2 2021. Nabota’s share in Daewoong Pharmaceutical's specialty drug sales exceeded 10% in Q3 2020 for the first time. After surpassing 20% in Q1 last year at 20.6%, it remained in the 10% range until Q1 this year. However, with the surge in Nabota’s sales, the drug accounted for more than a quarter of the company’s specialty drug sales in Q2. More recently, the company’s new drug Fexclue has also contributed significantly to company sales. In Q2, sales of Fexuclue more than doubled year-on-year to KRW 33.2 billion. Fexclue is a potassium-competitive acid blocker (P-CAB) drug that treats GERD. Fexuclue was approved by the MFDS in December 2021 and began full-scale sales in July 2022 after being listed for reimbursement on Korea’s health insurance benefit list. Nabota and Fexuclue jointly generated KRW 86.3 billion in sales in Q2, accounting for 39.6% of the company's specialty drug sales. Daewoong Pharmaceutical set a new performance record thanks to the steep growth of its self-developed drugs. On a standalone basis, Daewoong Pharmaceutical's Q2 revenue increased 6.0% year-on-year to KRW 325.5 billion, and operating profit increased 37.1% to KRW 49.6 billion. Both the revenue and operating profit in Q2 were the largest in the company's history. The operating profit margin as a percentage of sales was 15.2%, a significant improvement from the 11.8% it had made in the same period of the previous year, showing high performance. Daewoong Pharmaceutical's operating profit on a consolidated basis was KRW 42.3 billion in Q2, up 5.6% year-on-year, the largest ever. Revenue increased 3.0% year-on-year to KRW 360.5 billion, the second-highest amount ever recorded after Q4 of last year.
- Company
- 'High immunity vs. high dose’ flu vaccine for older adults
- by Hwang, Byung-woo Aug 13, 2024 05:47am
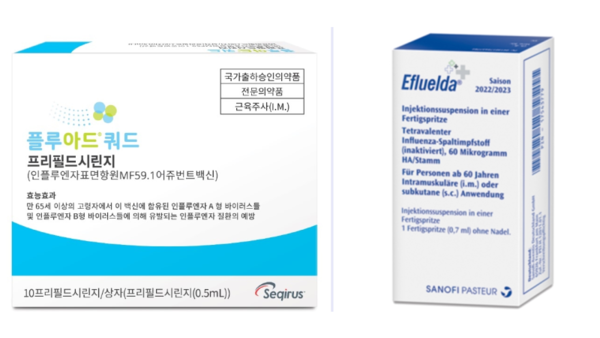
- Equipped with their respective flu vaccines specialized for people aged 65 and older, Sanofi and CSL Seqirus are seeking to expand their market share in Korea’s influenza (flu) vaccine market, which is driven by vaccines registered in the National Immunization Program (NIP). CSL Seqirus was the first to enter this market last year, followed by Sanofi, which newly entered the market this year. The companies will face off for the first time in the 2024-25 flu season. Each is expected to target, highlighting their respective characteristics, high immunogenicity and high dose. (From the left) Pic of Fluad Quadrivalent, Efluelda According to industry sources on the 13th, ‘Efluelda,’ Sanofi’s high-dose influenza vaccine for people aged 65 and older, will be launched in time for this year’s flu season. Efluelda contains 4 times more the amount of antigens compared with conventional vaccines and has a mechanism of action that elicits a stronger immune response, preventing 24.2% more infection compared to the standard-dose vaccine. Previously, CSL Seqirus’s Fluad Quad was the first flu vaccine to be launched for people aged 65 and older. It was approved by the Ministry of Food and Drug Safety in 2022 and launched in time for the 23/24 season last year. Fluad Quad is a quadrivalent influenza vaccine that includes the immune booster 'MF59' and was developed by adding one type of influenza B virus to the company’s existing trivalent influenza vaccine Fluad. It offers improved size and breadth of immune response through the immune booster in older adults. The subsequent entry of Efluelda into the market, which was preemptively occupied by Fluad Quad, has ignited competition in the 65+ age group. While the inclusion of the influenza vaccine in the NIP could be a hurdle, the two companies believe there is an unmet need for flu protection amongst the elderly in Korea. In fact, despite the high rates of influenza immunization among people aged 65 and older, two-thirds of all influenza deaths in Korea occur in people 60 and older. In particular, older adults are known to be particularly vulnerable to influenza infection and complications due to decreased immune function and comorbidities. They also receive less protection after vaccination than younger adults. "While the majority of influenza vaccinations for people aged 65 and older are administered through NIP, the company believes there is still significant market development potential given the lower vaccine efficacy and risk of complications in older adults," said a Sanofi representative. "The need for high immunogenic influenza vaccination option has been increasing amongst older adults in Korea, with the Korean Society of Infectious Diseases' revision to the Adult Immunization Guideline last year recommending high immunogenic influenza vaccination for those aged 65 and older," said a CSL Seqirus representative. Fluad Quad was approved in 2022 and launched on the market last year Flu vaccination for older adults enters NIP...companies ponder over private market marketing challenges NIP However, due to the high share of NIP vaccinations amongst total influenza vaccinations, the private, non-reimbursed market for those aged 65 and older is limited. As a result, both Sanofi and CSL Seqirus would need to highlight the value of their vaccines, while holding in check the competition between products. In its second season on the market, Fluad Quad is expected to be available in the country from mid-September, at a similar volume to last year. CSL Seqirus’s marketing keyword for this season is 'filial piety'. The company is focusing on communicating the differentiated superiority of Fluad Quad compared to the existing egg-based vaccine and is planning activities to express the feelings of sons and daughters who care about their parents' health. In particular, before the launch of the influenza vaccine, the company emphasized its strengths over its competitor, announcing the results of a study on the relative vaccine effectiveness (rVE) of trivalent immune-boosting influenza and trivalent high-dose influenza vaccines. "While high-dose influenza vaccines simply increase the amount of antigen, Fluad, an immune-boosting influenza vaccine, is different as it improves the immune response through MF59, an adjuvant developed through our company’s proprietary technology, to enhance the prevention effect," emphasized a CSL Seqirus representative. While Sanofi did not disclose specific volumes, the company said it is preparing for full-scale marketing activities, including national lot release, for Efluelda. The marketing keyword for Efluelda is "flu prevention, more than just protection" and will focus on its ability to address the unmet needs of older adults in terms of flu infections and hospitalizations from complications. "We are taking a deliberate and systematic approach to address the unmet need for flu prevention in the elderly," said a Sanofi representative. "We are planning a range of promotional activities targeting healthcare providers as well as consumers, as the high-dose vaccine has been shown to provide greater protection and reduced hospitalization rates compared with conventional vaccines."
- Company
- Hanmi’s Rolvedon posts sales of KRW 20.6 billion in Q2
- by Son, Hyung-Min Aug 13, 2024 05:47am
- Sales of Rolvedon (Korean brand name: Rolontis), a new anti-cancer drug developed by Hanmi Pharmaceutical, are showing signs of recovery in the U.S. market. Hanmi Pharmaceutical's U.S. partner Assertio plans to increase Rolvedon’s market share through new clinical trials that could ensure the drug’s advantage in convenience of administration. According to Assertio on the 12th, Rolvedon generated USD 15.1 million in U.S. market sales in Q2 this year, down 28.1% YoY, but up 4.1% from the previous quarter. Rolvedon is a neutropenia treatment developed by Hanmi Pharmaceutical and was approved as the 33rd homegrown new drug in Korea in March 2021. Hanmi Pharmaceutical and its U.S. partner Spectrum (now Assertio) obtained U.S. Food and Drug Administration (FDA) approval for Rolvedon in September 2021. Hanmi Pharmaceutical transferred Rolvedon’s technology to the U.S. company Sepctrum in 2012. Assertio acquired Spectrum and Rolvedon in April last year. Assertio specializes in the development of inflammation treatments, including the non-steroidal anti-inflammatory drug indocin and the orally disintegrating film-based drug Sympazan. The company sought to enter the anticancer market with Rolvedon. The drug is administered to prevent or treat neutropenia in cancer patients who receive myelosuppressive chemotherapy. As a granulocyte colony-stimulating factor (G-CSF) class drug, it stimulates the granulocyte to increase neutrophil production, similar to ‘Neulasta (pegfilgrastim).’ Changes in quarterly sales of Rolvedon (Unit: USD 1 million) Since its launch in October 2022, the drug generated USD 10.1 million in Q4 sales. In December of the same year, Rolvedon was included in the National Comprehensive Cancer Network (NCCN) febrile neutropenia prevention and treatment guidelines. Since then, Rolvedon’s sales continued to grow, generating USD 15.6 million in Q1 and USD 21 million in Q2 last year. Rolvedon’s sales growth slowed in Q3 last year, posting sales of USD 8 million, a 62.0% decrease from the previous quarter. The decline was primarily due to new reimbursement terms applied to Rolvedon. The new reimbursement system, which has been in place since April last year, is reportedly less favorable than the terms that were applied upon its launch. Rolvedon’s sales rebounded in Q4 last year, generating USD 10 million in revenue, and its sales continued to grow in Q1 and Q2 of this year, posting USD 14.5 million and USD 15.1 million, respectively. Assertio attributed the rebound to new accounts won with its new commercialization strategy. Since its launch in the U.S. in Q4 2022, Rolvedon has generated USD 95.3 million (KRW 130 billion) in cumulative sales. Company completes patient enrollment for a same-day dosing clinical trial for Rolvedon...to compete with Neulasta Assertio made a bid to Rolvedon’s competitor Neulasta with a same-day dosing trial. One of the disadvantages of using neutropenia treatments like Neulasta is that the drug can't be administered until 24 hours after chemotherapy, which increases the number of hospital days for patients. Amgen and Kyowa Kirin, the developers of Neulasta, are defending the market with the launch of Neulasta Onpro, which is designed to be worn on the day of chemotherapy and automatically deliver Neulasta the next day. Neulasta OnPro is designed to be attached to a patient's body the same day they receive chemotherapy, and then automatically deliver the drug the next day. Assertio plans to compete with Neulasta Onpro with a same-day dosing trial of Rolvedon. Assertio is currently enrolling patients in its Phase I same-day dosing trial for Rolvedon. Assertio aims to present initial same-day clinical data at an international academic conference within the year.
- Company
- JAK inhibitors market size rose 54%↑over the year
- by Kim, Jin-Gu Aug 12, 2024 05:55am
- (Clockwise from upper left) product photos of Jyseleca, Cibinqo, Rinvoq, Olumiant, and Xeljanz. The market for Janus Kinase (JAK) inhibitors, oral medicines used to treat autoimmune diseases, is growing rapidly following the approval of major medicines for expanded indications. In the first half of the year, the market for JAK inhibitors reached KRW 27.5 billion in outpatient sales, up 54% year over year (YoY). Abbvie's 'Rinvoq (upadacitinib)' occupies the No.1 slot among the major products with a considerable margin over other products. Lily's 'Olumiant (baricitinib),' Pfizer's 'Xeljanz (tofacitinib)'·'Cibinqo (abrocitinib),' and Eisai's 'Jyseleca (filgotinib)' are increasing prescription performance. The JAK inhibitors market grew 54% over the year…market size expected to be worth KRW 50 billion by the end of 2024 According to the pharmaceutical market research firm UBIST on August 9th, the outpatient market size for JAK inhibitors in the first half of 2024 is worth KRW 27.5 billion, up 54% YoY compared to KRW 17.8 billion last year. JAK inhibitors are medicines used to treat autoimmune diseases, including rheumatoid arthritis and atopic dermatitis. They work inhibiting inflammatory cytokines, which in turn inhibit inflammation, pain, and cell activation. After the launch of Xeljanz in 2015, Olumiant and Rinvoq were introduced to the race in 2019 and 2021, respectively. Cibinqo and Jyseleca followed last year. South Korea JAK inhibitors market outpatient prescription sales (unit: KRW 100 million, source: UBIST). Eisai’s Jyseleca (red), Pfizer’s Cibinqo (purple), Abbvie’s Rinvoq (light blue), Lily’s Olumiant (blue), and Pfizer’s Xeljanz (dark blue). After new drugs launched one after another, the market is expanding rapidly. JAK inhibitors market worth KRW 12.5 billion in 2019 has expanded to KRW 18.7 billion in 2020, KRW 25.5 billion in 2021, KRW 33.5 billion in 2022, and KRW 40 billion last year. The market recorded KRW 27.5 billion just for the first half of this year and is expected to top KRW 50 billion by the end of 2024. The prescription sales of the market leader Rinvoq doubled over the year…a substantial margin compared to No.2 and No.3 Among major products, Rinvoq is at the top of the ranking. The prescription sales of Rinvoq for the first half of the year amounted to KRW 11.2 billion, a 2.2-fold increase from KRW 5.2 billion YoY. Following Rinvoq, Olumiant ranked No.2 with KRW 8 billion in the first half of the year. It has seen a 28% increase over the year compared to KRW 6.2 billion YoY. During the same period, the prescription sales of Xeljanz increased 8%, from KRW 6.4 billion to KRW 6.9 billion. These three products competed against each other for the No.1 slot. Xeljanz, the first drug launched, was a market leader until the first quarter of last year. However, Olumiant surpassed Xeljanz and occupied the No.1 slot in the second and third quarter of last year, with a marginal difference. Quarterly prescription sales of JAK inhibitors (unit: KRW 100 million, source: UBIST). Xeljanz (dark blue), Olumiant (blue), and Rinvoq (light blue). From the fourth quarter of last year, Rinvoq's sales grew rapidly. Rinvoq's prescription rose substantially in the fourth quarter of last year, and Rinvoq, which used to rank No.3, became No.1. Furthermore, its prescription sales increased by a greater margin in first and second quarters of this year. Rinvoq has surpassed the prescription sales of Olumiant and Xeljanz, dominating the market. Rinvoq experienced rapid growth in sales following approval for "adolescent's atopic dermatitis' indication…in progress for adding 'children's atopic dermatitis' Analysis suggests that expanded indication have contributed to Rinvoq's rapid growth, especially after it was approved for the indication to treat adults and adolescents 12 years of age and older with atopic dermatitis. Rinvoq is indicated for the treatment of ▲rheumatoid arthritis, ▲psoriatic arthritis, ▲ankylosing spondylitis, ▲atopic arthritis, ▲ulcerative colitis, and ▲Crohn's disease. Olumiant is indicated for the treatment of ▲rheumatoid arthritis, ▲atopic dermatitis, and ▲alopecia areata. Xeljanz is indicated for the treatment of ▲rheumatoid arthritis, ▲psoriatic arthritis, and ▲ankylosing spondylitis. Rinvoq and Olumiant's increased prescription sales were driven by atopic dermatitis. However, these two differ in the range of usage. Rinvoq can be used to treat adults and adolescents of 12 years and older with moderate to severe atopic dermatitis, while Olumiant can be used to treat adults with moderate to severe atopic dermatitis. Additionally, a Phase 3 clinical trial for Rinvoq is in progress, targeting children of 2 to 12 years with atopic dermatitis. When the clinical trial concludes and receives approval for an expanded indication, Rinvoq's prescription sales will rise even higher. Cibinqo and Jyseleca, launched last year, are growing in market impact. Pfizer launched Cibinqo with reimbursement coverage in July of last year, as a Xeljanz follow-up. Cibiqo recorded KRW 700 million in prescription sales in last year's second half and generated KRW 1.2 billion in this year's first half. Cibinqo received approval for the indication to treat atopic dermatitis, the market dominated by Rinvoq and Olumiant. It is expected to have rapid growth, considering that it secured an indication to treat moderate to severe atopic dermatitis in adults and adolescents aged 12 years or older, similar to Rinvoq. Jyseleca was launched in November of last year as the fifth JAK inhibitor. It is indicated for the treatment of rheumatoid arthritis and ulcerative colitis. For the first half of 2024, the prescription sales for Jyseleca were KRW 300 million.
- Company
- Columvi can be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Aug 12, 2024 05:55am
- ‘Columvi,' the first bispecific antibody treatment option for lymphoma, may be prescribed at general hospitals in Korea. According to industry sources on the 9th, Roche Korea's CD20-CD3 bispecific antibody for diffuse large B-cell lymphoma (DLBCL) Columvi (glofitamab) passed Samsung Medical Center’s drug committee review. However, Columvi is currently a non-reimbursed drug. Its reimbursement application was reviewed by the Cancer Disease Deliberation Committee in July but was unable to set reimbursement standards at the time. Whether the company will quickly reorganize and reapply for coverage is gaining interest. Columvi was approved in Korea last December for the treatment of adult patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL), after two or more lines of systemic therapy. The drug is a third-line treatment option for DLBCL, like Novartis’s chimeric antigen receptor (CAR)-T-cell therapy Kymriah (tisagenlecleucel). The two drugs have different benefits; therefore the choice will likely be based on each patient's condition and circumstance. Columvi demonstrated efficacy in the Phase I/II NP30179 trial in 155 patients with relapsed or refractory DLBCL after two or more prior systemic therapies. Results showed that Columvi achieved a complete response (CR) of 40% and an overall response rate(ORR) of 52%. The efficacy was also consistent across all subgroups. The most common adverse event was cytokine release syndrome (CRS). Adding to the encouraging data, at the 2024 Congress of the European Hematology Association (EHA 2024), the company unveiled the results of the Phase III STARGLO study, which demonstrated an improvement in overall survival (OS) with Columvi. The STARGLO study enrolled patients with relapsed or refractory (R/R) diffuse DLBCL who were not eligible to receive an autologous stem cell transplant after one or more prior systemic therapies, or who had received two or more prior systemic therapies. In the primary analysis (median follow-up 11.3 months), Columvi and gemcitabine+oxaliplatin (GemOx) combination significantly improved the primary endpoint of OS with a 41% lower risk of death compared to rituximab+GemOx. Seok Jin Kim, Professor of Hematology and Oncology at Samsung Medical Center, said, "There had been much unmet need in DLBCL for more effective third-line treatment options for patients who fail first-line or experience repeated relapses. We expect the introduction of Columvi to significantly improve the outcomes for patients with relapsed or refractory lymphoma in Korea."
- Company
- Anticancer drug 'Alecensa' expected to win nod
- by Eo, Yun-Ho Aug 12, 2024 05:55am
- Anticancer drug The ALK anticancer drug Alecensa is anticipated to receive approval for an additional indication as an adjuvant therapy for treating lung cancer. Sources said that the review for indication approval by the Ministry of Food and Drug Safety (MFDS) is in its final stage for Alecensa (alectinib), Roche Korea's anaplastic Lymphoma Kinase (ALK)- targeted anticancer agent, as a post-operative adjuvant therapy for patients with early-stage non-small cell lung cancer (NSCLC). The indication of Alecensa has been approved worldwide, including by the U.S. FDA in April and the European Commission (EC). The basis of indication for the adjuvant therapy for early-stage NSCSC was the Phase 3 ALINA study, which demonstrated the efficacy. The study compared Alecensa to platinum-based chemotherapy to confirm the potential use of the drug as post-operative chemotherapy for treating patients with early-stage NSCLC. The trial enrolled 257 patients with stage IB to IIIA ALK-positive NSCLC who are 18 years old and older. The patients were randomly assigned to the Alecensa 600 mg treatment group (twice daily) and the platinum-based chemotherapy treatment group (max. 4 times every 3 weeks). The primary endpoint of the study was set as disease-free survival (DFS), and the secondary endpoints were overall survival (OS), the Central Nervous System-DFS (CNS-DFS), and the safety. The clinical results showed that the Alecensa treatment group had a 93.8% DFS at 2 years, which was higher than the 63% DFS of the platinum-chemotherapy treatment group. The 2-and 3-year CNS-DFS for the Alecensa treatment group were 98.4% and 95.5%, respectively. These measures were higher than the respective rates of 85.8% and 79.7% for the platinum-based chemotherapy treatment group. Furthermore, the Alecensa treatment group had a reduced risk of CNS disease progression or death by 78%. Meanwhile, adjuvant therapy with 'Tagrisso (osimertinib)' is commonly used to treat EGFR-positive NSCLC based on the ADAURA study, which demonstrated a superior effect over the platinum-based chemotherapy. Since Alecensa has shown to be more effective than the platinum-based chemotherapy, its use in clinical practice is expected to be widespread.
- Company
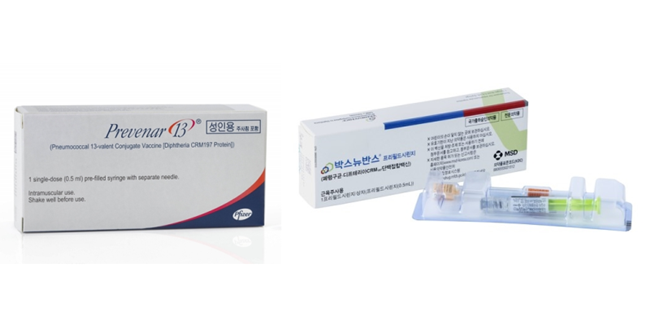
- Prevenar maintains lead in pneumococcal vaccine mkt
- by Hwang, Byung-woo Aug 12, 2024 05:54am
- The pneumococcal vaccine market, dominated by Pfizer's Prevenar 13, is now facing competition with the introduction of MSD's Vaxneuvance. The Q2 share report, which is the first report issued after Vaxneuvance’s introduction in April, showed that while Pfizer's Prevenar 13 sales remained strong, Vaxneuvance gained a larger share of the non-reimbursed adult pneumococcal vaccine market. (from the left)Pic of Prevenar 13, Vaxneuvance Prevenar 13 was the dominant pneumococcal vaccine in Korea’s market prior to the introduction of Vaxneuvance. It generated KRW 45.8 billion in sales last year (IQVIA data), and the drug occupied 98.9% in March 98.9% among children and 100% among adults (UBIST data). However, since the launch of Vaxneuvance in April, the market’s focus of interest has been on how the emergence of the Prevenar 13’s competitor. The first report card issued in Q2 can be summarized as Prevenar 13’s solid defense despite the strong advance made by Vaxneuvance. According to UBSIT, Prevenar 13 led the pneumococcal vaccine market in June last year, occupying 69.2% of the pediatric pneumococcal vaccine market. The company's market share declined from 99.1% in March to 83.5% in April and then to 77.4% in May, but is still holding the majority of the market. Prevenar 13’s market share (Source: UBIST, Pic: Dailpharm) The gap widens when considering the adult pneumococcal vaccine market. After reaching an 87% share in April, Prevenar 13’s share had dropped to 77% in May, but rebounded to 80% in June, showing even greater market dominance than in the pediatric pneumococcal vaccine market. The industry has linked this to Vaxneuvance’s rapid registration into the National Immunization Program (NIP). Vaxneuvance entered the pediatric NIP within a month of its approval late last year and was covered through the NIP upon its launch. As a result, MSD focused on the pediatric market launch, using up most of its initial supply for NIP. The company's strategy was to aggressively target the NIP market, where it can expect stable sales. At a media seminar held on August 6, MSD said that it has been gaining a double-digit share every month. While the pediatric NIP market is likely to remain competitive, the adult market, where Prevenar 13 has an 80% share, is likely to be a concern for MSD. Contrary to how the NIP market is less commercially driven, the off-patent market is more likely to reflect the company’s marketing capabilities. Prevenar 13 and Vaxneuvance’s price competition on siteThis means that MSD would need to overcome Prevenar 13’s long-standing recognition among physicians and patients, with the cost of vaccination also serving as a competitive factor. It may be difficult for Vaxneuvance, which is relatively new to the market, to compete on price. MSD is emphasizing that Vaxneuvance has 2 more - 22F and 33F - unique serotypes compared with Prevenar 13 and with confirmed good immunogenicity. The Korean Society of Infectious Diseases recently recommended Vaxneuvance as a priority in the revised 2024 Adult Vaccination Guidelines. However, how the doctors will regard the difference of adding the 2 serotypes - 22F and 33F - in practice will be key, as the 10A serotype is found more in children and 3 and 19A serotypes in adults in Korea, and 19A and 19F serotypes are the most frequent serotypes among serotypes preventable through children’s vaccines. The next variable in the competition between the 2 vaccines will be Pfizer's follow-on vaccine, Prevenar 20. Industry insiders expect Prevenar 20 may be approved later this year and launched at the end of the year at the earliest. Although the new vaccine will not be able to influence the NIP market or guidelines in the short term, from the perspective of Vaxneuvance, the emergence of Pfizer's follow-up vaccine less than a year after its market launch can rise as a concern when expanding its share. Regarding this, a Pfizer representative said, "It is difficult to predict the timing of Prevenar 20’s approval, but we are working to launch the vaccine as soon as possible."
- Company
- Rare disease drug Ilaris is reimbursed 9 yrs after approval
- by Hwang, Byung-woo Aug 11, 2024 04:46pm
- Expectations are rising in the field for the hereditary recurrent fever syndrome Ilaris (canakinumab), which was granted reimbursement 9 years after approval. As a treatment for an extremely rare disease with a small number of patients, its reimbursement is expected to address unmet needs in an area with no treatment option. However, due to the nature of rare diseases, many patients suffer through a diagnostic odyssey, so efforts to diagnose patients quickly, such as by improving disease awareness, would be necessary to maximize the reimbursed use of Ilaris. Novartis Korea held a press conference on the 8th to highlight the implications of the health insurance reimbursement coverage of Ilaris for hereditary recurrent fever syndromes (CAPS, TRAPS, FMF) Novartis Korea held a press conference on the 8th to highlight the implications of Ilaris’s reimbursement coverage for hereditary periodic fever syndrome (CAPS, TRAPS, FMF) in Korea. Hereditary periodic fever syndromes are a group of rare auto-inflammatory diseases characterized by periodic episodes of unexplained fever and rashes throughout the body, including cryopyrin-associated periodic syndromes (CAPS), tumor necrosis factor receptor-associated periodic syndrome (TRAPS), and familial Mediterranean fever (FMF). In addition to ▲CAPS, ▲TRAPS, and ▲FMF, Ilaris can be prescribed in Korea for ▲hyperimmunoglobulin D syndrome/mevalonate kinase deficiency (HIDS/MKD) and ▲systemic juvenile idiopathic arthritis (JIA). In the case of the CAPS indication, it is further categorized into the following symptoms: ▲Familial cold autoinflammatory syndrome (FCAS)/ familial cold urticaria (FCU) ▲Muckle-Wells syndrome (MWS) ▲Neonatal onset multisystem inflammatory disease (NOMID)/chronic infantile neurological, cutaneous and articular syndrome (CINCA). "All three of these indications are extremely rare diseases, and many patients suffer through a diagnostic odyssey that prevents them from receiving an accurate diagnosis," said Dae-Chul Jeong, professor of Pediatrics at Seoul St. Mary's Hospital, "even after diagnosis, their treatment options were very limited, which was frustrating for them and us as medical professionals." Dr. Dae-Cheol Jung, Professor of Pediatrics, Seoul St. MaryIn this situation, Professor Jeong believes that the long-awaited Ilaris's reimbursement approval will enable higher utilization of the drug. Ilaris is an interleukin-1 (IL-1) inhibitor recommended for the treatment of CAPS in the 2021 international guidelines of the European Congress of Rheumatology and the American College of Rheumatology. In a Phase III study, the drug demonstrated significant clinical benefit in remission after a single dose and remission rate at 6 months and can be administered every 8 weeks in patients with CAPS, improving the quality of life for patients and their caregivers. In the CLUSTER study, which included 46 patients with TRAPS and 63 patients with colchicine-resistant FMF, 45% (n=10/22) of TRAPS patients treated with Ilaris 150 mg and 61% (n=19/31) of colchicine-resistant FMF patients achieved a complete response at week 16. "With the reimbursement of Ilaris, patients with hereditary recurrent fever syndrome who had limited treatment options will be able to benefit from the use of standard therapy," said Keun-Sung Lee, Director of Medical Affairs at Novartis Korea. "The reimbursement approval is significant as it will allow patients and their families to benefit from the improved quality of life by reducing the number of dosing required, as these diseases require long-term treatment.” Also, the importance of diagnosis in order to access the benefits of Ilaris had been emphasized at the conference, as it is a treatment for extremely rare diseases. This means that efforts need to be made to prevent the so-called “diagnostic odyssey” of the patients. "Publicity about the disease is the most needed, and we believe that we can raise awareness about the disease through symposiums at the society level,” said Professor Jeong, “I think that the introduction of genetic testing will further increase diagnosis.” "Since NGS is performed in most university hospitals, the number of tests is increasing in line with the awareness of the disease," he added, "There are problems in FMF diagnosis in adults, so we need to raise awareness step by step, such as by promoting it to internal medicine specialists."
- Company
- Yuhan acquires new drugs from biotech venture firms
- by Chon, Seung-Hyun Aug 09, 2024 05:34am
- Yuhan significantly increased its research and development (R&D) investment. Over the past five years since acquiring new drug technology from biotech venture companies, Yuhan's R&D investment size has reached its peak. This expansion of outward investment seems to be the company's outlook for securing a new portfolio. According to Yuhan on July 31st, the company's R&D cost in Q2 amounted to KRW 53.5 billion, up 39.8% from KRW 38.2 billion year over year (YoY). It increased 17.0% from the previous quarterly investment amount of KRW 45.7 billion. Yuhan's quarterly R&D investment amount exceeded KRW 50 billion in four years since Q4 2020. In 2020, its R&D investment spending significantly increased due to conducting the global Phase 3 trial for the new anticancer drug Leclaza monotherapy. Yuhan's quarterly R&D investment amounts (unit: 1 million, source: Yuhan). Yuhan's expanded R&D investment for this year is attributed to acquiring promising technologies from biotech venture companies. In Q2, Yuhan invested KRW 8 billion in two biotech ventures. In March, Yuhan signed contracts with Cyrus Therapeutics and KANAPH Therapeutics to acquire technology transfer of anticancer drug candidates based on the SOS1 inhibitor mechanism. The contract value amounted to KRW 208 billion, including milestones for future development, approval, and sales. Cyrus Therapeutics is a biotech venture developing targeted anticancer drugs and targeted protein degraders through medicinal and pharmaceutical chemistry-based technology. KANAPH Therapeutics is developing the next-generation new drug for the field of cancer and autoimmune diseases based on pharmaceutical convergence technology. The SOS1 inhibitors that Yuhan acquired are anticipated to increase treatment effectiveness in synergy with KRAS inhibitors or EGFR inhibitors and help solve tolerance to conventional therapies. SOS1 is a protein regulating the activity of RAS, involved in cell proliferation, and it is regarded as a promising target for anticancer function regardless of various RAS mutant types or cancer types. KRAS and EGFR mutations are common causes of lung cancer, colorectal cancer, pancreas cancer, and cancers with unmet medical needs. Targeting these is expected to have significant potential in terms of the market. Cyrus Therapeutics and KANAPH Therapeutics discovered non-clinical candidate products through joint research. At the American Association for Cancer Research (AACR) conference last year, they presented results showing advantages in pharmacological properties, including superior anti-cancer efficacy in xenograft animal models compared to competitive drugs and improved drug dynamics within the body. In the second quarter, Yuhan Corporation paid KRW 3 billion in royalties to the biotech company J INTS BIO. J INTS BIO is focused on developing anticancer drugs. In 2021, J INTS BIO secured a new pipeline by entering into a transfer agreement for a novel drug candidate developed by Dr. Kwang Ho Lee from the Korea Research Institute of Chemical Technology (KRICT) and Professor Byoung Chul Cho, Director of the Lung Cancer Center at Yonsei Cancer Hospital. Yuhan entered into a partnership with J INTS BIO by investing KRW 20 billion each in equity in 2021 and 2022. Last May, Yuhan signed a licensing agreement with J INTS BIO for the targeted therapy 'JIN-A04.' Yuhan has secured exclusive global rights to develop and commercialize J INTS BIO's tyrosine kinase inhibitors that target HER2 and EGFR. The technology transfer agreement has a total contract value of KRW 429.8 billion, with a non-refundable upfront payment of KRW 2.5 billion. On August 5th, Yuhan received approval from the U.S. Food and Drug Administration (FDA) for the Phase 1/2 Investigation New Drug (IND) under the code name YH42946. The study will evaluate the safety, drug tolerance, pharmacokinetics, and anti-tumor activation following oral administration of YH42946 in patients with locally advanced or metastatic solid cancers harboring HER2 mutation and EGFR exon 20 insertion mutations. Yuhan focuses its R&D investment on acquiring external technology to uncover new growth opportunities. A prime example of Yuhan's success in open innovation is its cancer drug, Leclaza. Yuhan obtained the development rights for Leclaza from Oscotec and its subsidiary, Genosco, in 2016, just before the drug reached the preclinical stage. The total contract value was KRW 1.5 billion. Under the terms of the agreement, the partner received a fixed technology fee of KRW 1 billion within 30 days of the contract signing and an additional KRW 500 million upon approval of Phase 1 clinical trials. Yuhan entered into a global licensing agreement with Ubix Therapeutics for prostate cancer treatment in May. Ubix Therapeutics is a Korean biotechnology company developing new anticancer drugs. Yuhan secured global exclusive rights from Ubix Therapeutics to develop and commercialize a targeted protein degradation (TPD) agent that degrades androgen receptor. The contract value was up to KRW 150 billion. An upfront payment was KRW 5 billion, and Ubix Therapeutics could earn up to KRW 145 billion based on development, approval, and sales milestones. The contract also requires Yuhan to pay royalties based on the net sales of the drug it directly markets. Additionally, if Yuhan enters into a third-party technology transfer agreement for the product, the revenue from such a technology transfer will be distributed according to the drug's development stage when the agreement is signed.