- LOGIN
- MemberShip
- 2025-12-22 17:05:50
- Company
- Phesgo can be prescribed in Korea with reimbursement
- by Eo, Yun-Ho Sep 30, 2024 05:46am
- Breast cancer biobetter ‘Phesgo’ can now be prescribed in general hospitals in Korea with insurance reimbursement. According to industry sources, Phesgo (pertuzumab/trastuzumab), which is a subcutaneous injection combination of Roche's Perjeta and Herceptin, has passed drug committees (DC) reviews of tertiary hospitals in Korea, including Samsung Medical Center and Seoul National University Hospital. As a biobetter drug, the drug has been reimbursed since August, being applied to the preferential drug pricing plan. Phesgo is a combination of Herceptin and Perjeta, drugs that were previously administered intravenously, into a single subcutaneous injection. The primary benefit of the change from an intravenous to a subcutaneous formulation is its reduced administration time. When treating HER2-positive breast cancer, the existing intravenous regimen required a total of 270 minutes (4.5 hours) for a single dose administration and observation. In the case of Phesgo, the treatment can be completed within 20 minutes as it requires 5 minutes of administration and 15 minutes of observation time, reducing the time by up to 90% compared to traditional therapy. The coinsurance rate of Phesgo was set at the same as for Perjeta: ▲30% when administered in combination with chemotherapy as neo-adjuvant therapy for patients with locally advanced inflammatory or early-stage (>2 cm in diameter) HER2-positive breast cancer; ▲100% when administered in combination as adjuvant therapy for patients who are HER2-positive and have lymph node-positive breast cancer (up to 18 cycles of trastuzumab and pertuzumab combination therapy); and▲5% when administered in combination for patients with metastatic or unresectable locally advanced recurrent breast cancer who are HER2-positive and have not received prior HER2 therapy or chemotherapy. In the Phase III FeDeriCa study that studied 500 patients with HER2-positive early-stage breast cancer, the Phesgo subcutaneous arm was found to be non-inferior to the trastuzumab and pertuzumab intravenous arms. In general, because intravenous and subcutaneous injections have different routes of administration, their anticancer effect is identified based on the trough level and the probability of being cancer-free at the time of surgery. In the case of Phesgo, there was no difference in the trough level according to the route of administration, and the anti-cancer effect and survival period of the two therapies were the same, offering added strength of convenience to the existing advantages. “In the FeDeriCa study, Phesgo subcutaneous injection demonstrated equivalent trough levels as intravenous trastuzumab and pertuzumab,” said Seock-Ah Im, Professor of hematology-oncology at Seoul National University Hospital. ”It provides convenience to both patients and healthcare providers by reducing treatment time while maintaining the effectiveness and safety of intravenous trastuzumab and pertuzumab.” Meanwhile, the insurance authorities have also decided to reimburse Phesgo through the risk-sharing agreement scheme, to save health insurance finances. This is because Phesgo’s development target product, Perjeta, is also currently covered by the risk-sharing agreement scheme.
- Company
- K-Phama companies are targeting Southeast Asia
- by Heo, sung-kyu Sep 27, 2024 05:53am
- Korean pharmaceutical companies target Indonesia to establish a bridgehead for Southeast Asia market entry. Companies have invested in Indonesia by buidling manufacturing plants. The government and associations are also in support. According to pharmaceutical companies on September 20th, pharmaceutical companies, the government, and organizations are quickly establishing collaborative partnerships with Indonesia. Recently, public-private representatives, including KPBMA, Ministry of Food and Drug Safety (MFDS), KIMCo, and 15 Korean pharmaceutical and biotech companies, have visited Jakarta, Indonesia, to seek collaborative business opportunities with Indonesian Food and Drug Authority (BPOM) and local companies. The collaboration between pharmaceutical companies and the government is critical because the industry has shown interest in entering the Indonesian market. Korean pharmaceuticals are showing intersts due the growth in Indonesia market and government's support for in-house pharmaceutical development. Indonesia's pharmaceutical and biotech market is expected to grow quickly from KRW 13 trillion in 2022 to KRW 18 trillion in 2026. The analysis suggests that population growth and increased intractable diseases, including cancer and degenerative brain diseases, from an aging society will contribute to such development. Furthermore, Indonesia is considered a hub for pharmaceutical R&D·production·consumption. It is the biggest pharmaceutical consumption market among the countries in the Association of Southeast Nations (ASEAN). Additionally, to target the "Halal Belt," which consists of approximately 1.9 billion people worldwide due to population growth in Muslim countries, the Indonesian market is considered as a bridgehead in response to the increasing demand for "Halal certification" for pharmaceuticals. For these reasons, Korean pharmaceutical companies are currently establishing joint-subsidiaries with local companies, building manufacturing plants, and out-licensing technologies, in addition to exports. In particular, countries that have already entered the market are experiencing the results. Korean pharmaceutical companies that have entered the market include Daewoong Pharmaceutical, Chong Kun Dang, GC Biopharma, SK Plasma, HK inno.N, Il Dong Pharmaceutical, and Daewon Pharm. For Daewoong Pharmaceutical, the stem cell factory in the Cikarang Javabeka Industrial Complex of Daewoong Biologics Indonesia, an Indonesian subsidiary, obtained GMP certification from the Indonesian BPOM and began full-scale operation. Since establishing a branch in Jakarta in 2005, Daewoong Pharmaceutical has steadily strengthened cooperation, including establishing a joint venture with Infion, Daewoong Infion, in 2012. These efforts are bearing fruit. Chong Kun Dang established Indonesia's first halal-certified anticancer drug production plant in 2019 after establishing a joint venture, 'CKD-OTTO,' with the Indonesian pharmaceutical company OTTO in 2015. In addition, the Indonesian joint venture is achieving success by exporting products from Indonesia to Algeria and other countries. GC Biopharma received final approval for the business license for blood product plant construction and technology transfer from the Indonesian BPOM, on June 1, 2023. The company works with the Indonesian Red Cross and local pharmaceutical company P.T. Triman to supply plasma for blood product processing and plant business. A three-party business agreement has been signed concerning this. GC Cell, an affiliate of GC Biopharma, has signed a partnership agreement with the Indonesian company 'Kalbe Farma' and entered into a technology transfer and out-licensing agreement for 'Immune Cell LCD', a patient blood-derived immune anti-cancer cell therapy. The launch is aimed for next year. SK Plasma received approval from the Indonesian BPOM in March last year to build a plasma fractionation plant that can process 1 million liters of raw plasma annually. The company plans to establish a factory in collaboration with the 'Indonesia Investment Authority (INA)' and take charge of factory operations, business rights, production, and sales. In addition to companies establishing factories, HK inno.N exports K-CAB, Daewon Pharm exports Pelubi CR tab, and Boryung exports Kanarb tab. The government also continues to provide support. On September 11th, the MFDS dispatched a pharmaceutical entry support team through a public-private partnership. The MFDS visited Korean pharmaceutical companies operating in Indonesia to discuss the current status and outlook of the Indonesian market, learn about their experience in the local market, and collect information on other difficulties. The effort to enter the Indonesian market will continue through collaboration among government bodies, such as the MFDS, organizations, and pharmaceutical companies.
- Company
- 'Treatment options for psoriasis are evolving'
- by Son, Hyung Min Sep 27, 2024 05:53am
- Dr. April W. Armstrong, Professor and Chief of Dermatology at the University of California Los Angeles (UCLA) David Geffen School of Medicine “In the field of psoriasis, the development of many biologics has been largely abandoned due to the lack of convincing data showing similar efficacy to marketed drugs. On the other hand, in the field of oral drugs, interest in the TYK2 mechanism has increased upon the introduction of Sotyktu, increasing the industry’s R&D on such oral drugs. I expect to see an active oral psoriasis treatment option development in the coming years.” Dr. April Armstrong, a professor at the David Geffen School of Medicine at UCLA, recently spoke to Dailypharm about her rising expectations in the future of psoriasis treatment upon the introduction of various psoriasis treatment options. Professor Armstrong is an expert in the field of psoriasis, having served as chair of the Medical Board at the National Psoriasis Foundation and co-president of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Psoriasis is a chronic, non-contagious skin condition with flare-ups and remissions, with an estimated prevalence of 3% in Korea and an estimated 1.5 million patients. It is characterized by narrow, rice-like red patches on the skin, covered with white dead skin cells, which can grow from the size of a coin to the size of the palm of your hand when the rash becomes aggravated. Psoriasis is categorized by clinical type, and in “plaque psoriasis, which accounts for 80 to 90 percent of all cases, the rash is shaped like a plaque. Psoriasis is not just a skin disease, but a systemic and persistent immunologic abnormality that is difficult to cure and requires long-term management. The goal of treatment for psoriasis is to improve the quality of life by improving skin lesions and controlling flare-ups and exacerbations while minimizing the side effects of medications and preventing the many systemic complications caused by chronic inflammation. A variety of treatment options have already emerged for psoriasis, including TNF-α inhibitors and interleukin (IL)-inhibitors. In addition, new oral therapies, such as BMS' Sotyktu, have emerged to target both therapeutic efficacy and patient convenience. Sotyktu is the first TYK2 inhibitor approved for moderate-to-severe plaque psoriasis in adults. Sotyktu is taken orally once daily, regardless of meals, with no dosage adjustments, expanding the patients’ options in a setting where biologics were the only treatment option available outside of the existing universal treatment. Professor Armstrong emphasized that Sotyktu brought the psoriasis treatment environment a step forward and that oral formulations could be the next big R&D trend. Introductino of Sotyktu...first oral treatment option to target TKY2 Sotyktu is a TYK2 inhibitor. TYK2 is an important link in the signaling pathway of IL-23, a cytokine known to play a key role in the pathogenesis of psoriasis. TYK2 mediates multiple cytokine pathways, such as IL-23, which in turn triggers the production of pro-inflammatory cytokines including IL-17 that promote keratinocyte proliferation and epidermal overgrowth, resulting in psoriasis. As a TYK2 inhibitor, Sotyktu selectively binds to the regulatory domain of TYK2 and blocks IL-23 signaling, thereby reducing the production of IL-23 and IL-17, which in turn reduces keratinocyte proliferation. “Patients with psoriasis have long suffered from a lack of adequate treatments, and only recently have started gaining access to moderately effective treatments,” said Professor Armstrong. However, there is still an unmet need. Healthcare providers need to ensure that patients have access to treatments that fit their preferences and lifestyle. Some patients may be uncomfortable with the idea of injections. Some patients avoid Injectable medications due to significant anxiety, such as fear of needles, in which case oral medications can be an alternative.” The introduction of Sotyktu expanded options for patients who have primarily been prescribed injectables. In addition to the clinical trial that became the basis of its approval, Sotyktu’s efficacy and safety were confirmed in recently published 4-year patient follow-up results. The study, named POETYK PSO-1,2, LTE, looked at the Psoriasis Area and Severity Index (PASI) 75 response rate and static Physician's Global Assessment (sPGA) 0/1 response rate from 52 weeks to 4 years in 513 patients who continuously received Sotyktu for 4 years. Results showed that 71% of patients treated with Sotyktu maintained PASI 75 over the 4 years, and 57% of patients achieved and maintained a high standard of “clear” and “almost clear” skin. “No new adverse events or signals were identified in the safety data collected over the 4 years,” said Professor Armstrong. The safety profile and tolerability seen in the parent study were well maintained in the Sotyktu arm.” “In terms of safety, Sotyktu was free of serious or major adverse events, including major adverse cardiovascular events, cancer, and venous thromboembolism. Also, the serious adverse events that may arise with the Janus kinase (JAK) 1/2/3 inhibitors, such as serious infections and psoriatic arthritis, were not reported with the use of Sotyktu.” Another strength of Sotyktu is that it is effective in Asian patients. In the PSO-3 study, which focused on Asian patients from Korea, China, and Taiwan, the PASI response rate was approximately 10% higher than that identified in the global study. “The superior data of Sotyktu identified in the Asian study may be due to the fact that Asians tend to weigh less than Westerners, as well as genetic differences,” explained Professor Armstrong. “The PSO-4 clinical data in Japanese patients showed a 76% PASI 75 rate and 83% sPGA 0/1 rate at Week 16.” “However, it should be noted that both PSO-3 and 4 trials were relatively small studies compared to global studies, and PSO-4 was a single-arm study in Japanese patients. Single-arm studies without control arms tend to show higher rates,” added Professor Armstrong. Increased psoriasis treatment options... broaden choices for patients Treatment options for psoriasis have expanded with the introduction of a variety of new drugs, including injectable and oral therapies. Various treatment options, which include IL-17, IL-23, TNF-α inhibitors, and Sotyktu, are all improving the quality of life for patients with psoriasis. However, Professor Armstrong suggests that the future trend in R&D for psoriasis treatments will likely shift toward oral agents. “With the introduction of Sotyktu, the development of biologics (injectables) in the treatment of psoriasis has gradually faded away,” said Professor Armstrong, ”Fewer new biologics are being developed, except for those that are nearing the end of development or have already been launched in other countries and are expected to be introduced to Korea.” According to Professor Armstrong, some biologics are under clinical trials in the U.S., but many of them have been discontinued due to the lack of convincing data, showing similar efficacy to drugs already on the market. In the case of IL-17 inhibitors, concerns have been raised about the exacerbation of inflammatory bowel diseases such as ulcerative colitis and Crohn's disease, as well as oral candidiasis. IL-23 inhibitors have not been associated with any significant adverse events, but there have been concerns about pain or discomfort at the injection site and upper respiratory tract infections. On the other hand, the safety of oral Sotyktu is considered to be well-established with 4 years of data. While other oral therapies have been associated with higher rates of adverse events such as nausea and vomiting than placebo, Sotyktu has been well tolerated, with rates of these events not significantly different from placebo, said Professor Armstrong. “This is an exciting time for patients with psoriasis, due to the increasing treatment options available. Contrary to how patients had to choose between safety or efficacy when opting to use oral treatment options, oral therapies have evolved since then. New oral drugs such as Sotyktu have demonstrated long-term efficacy and safety comparable to first-generation biologics such as TNF-α inhibitors and IL-12/23 inhibitors,” said Professor Armstrong. ”The treatment options have significantly improved than in the past.”
- Company
- KRPIA expresses concerns over drug approval fee hike
- by Eo, Yun-Ho Sep 27, 2024 05:53am
- “The pharmaceutical industry is bound to feel burdened by the sudden decision. We hope that the new drug approval system will be adjusted through discussions with the industry.” The Korean Research-based Pharmaceutical Industry Association (KRPIA) expressed the pharmaceutical industry's stance regarding the amendment to the 'Fee Regulations for Approval of Drugs, etc.' that was announced by the Ministry of Food and Drug Safety (MFDS) on the 9th. As part of the ‘Measure for Innovation in New Drug Approval', the MFDS is expected to dramatically increase the fee for new drug approval from KRW 8.83 million to KRW 410 million, which is a nearly a 50-fold increase, by fully applying the benefit principle. In essence, KRPIA agrees with the MFDS’s proposal, including the need to realize new drug approval fees, strengthen review capabilities, and shorten the approval period. KRPIA saw that the MFDS’ decision to significantly increase the approval fee reflects the authorities’ intention to innovate the new drug approval process to respond more quickly and flexibly to environmental changes and meet new industrial demand. However, it added the industry’s concerns regarding the fee burden, which rose significantly - over 50 times - with the sudden announcement of the revision without a grace period or phased application. As this is an unprecedented increase in the fee, the KRPIA’s position is that an approval system and administrative services should be prepared at a level that everyone can agree on through sufficient discussions with the industry. “The license fee of KRW 410 million is very high compared to almost all developed countries except the U.S. and Europe,” KRPIA said, noting that Korea's market size is only one-fourth and drug prices are only 60% of Japan's, which also has a similar fee level. With many countries racing to introduce new drugs quickly to improve patient access to treatment, Korea's pharmaceutical market size, challenging drug price environment, and Korea-specific approval requirements suggest that an excessive approval fee hike could be another factor that can slow the introduction of innovative new drugs with low prevalence or small market size. “Its implementation in January 2025 may be too soon for pharmaceutical companies to prepare for changes, and seems to be an insufficient time for the MFDS to recruit specialized personnel and overhaul the system,” said KRPIA. “In order for the intention of the system to be well realized, the fee hike must be accompanied by an overhaul of the new drug approval system and the introduction of fast and advanced administrative services," emphasized KRPIA.
- Company
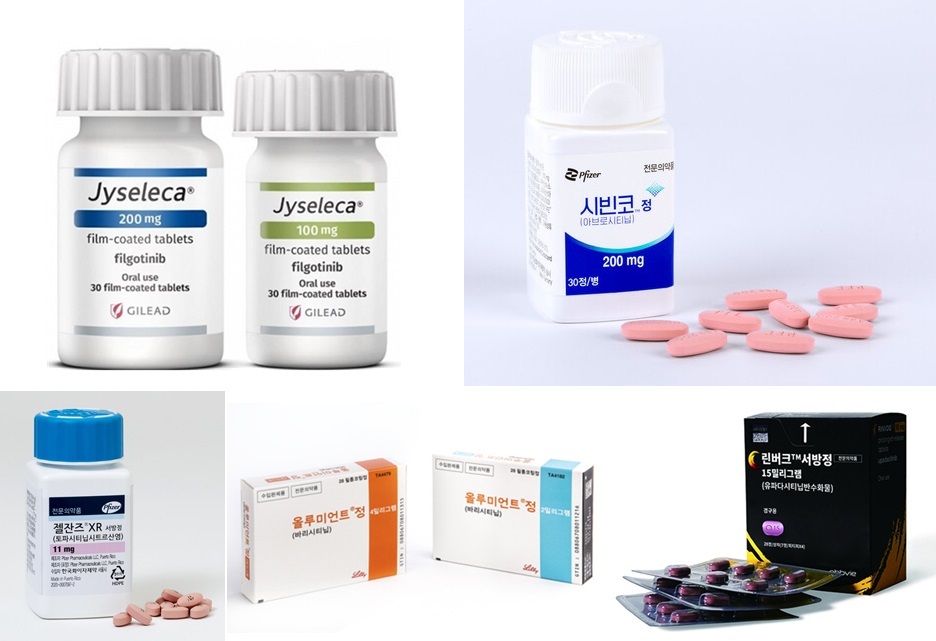
- Switching between JAKis for RA yet to be reimbursed
- by Eo, Yun-Ho Sep 27, 2024 05:52am
- 국내 처방되고 있는 JAK억제제들The plan to allow insurance reimbursement when switching between JAK inhibitors in rheumatoid arthritis, which was expected to take effect in October, has been postponed. According to Dailypharm coverage, the health authorities have recently put on hold the notification of the proposed revision that allows switching between JAK inhibitors in rheumatoid arthritis. The tentative timeline for the notification was set as the end of the year, but this is not confirmed yet. The delay was reportedly caused by problems with the final approval of the budget bill. Currently, 4 drugs are prescribed for rheumatoid arthritis in Korea, including Pfizer Korea’s Xeljanz (tofacitinib), Lilly's Olumiant (baricitinib), AbbVie's Rinvoq (upadacitinib), and Eisai Korea’s Jyseleca (filgotinib). Until now, the government has been adhering to the position that it is difficult to reimburse switching JAK inhibitors due to the lack of clinical evidence. However, after continuous statements submitted by the Korean College of Rheumatology and other organizations, as well as prescription experience on cross-dosing, the government reconsidered its position and came to a positive conclusion. The industry expected the change to be applied in October, but the notification of the amendment has been delayed. As a result, it remains to be seen if patients will be able to freely switch between JAK inhibitors before the end of the year. If reimbursement for switching between the drugs is allowed, such use is expected to further activate the JAK inhibitor market. According to drug market research firm UBIST, the outpatient prescription market for JAK inhibitors was KRW 27.5 billion in the first half of last year. This is a 54% increase in one year compared to the KRW 17.8 billion in the first half of last year. The market for JAK inhibitors has been expanding at a rapid pace. The market, which had been around KRW 12.5 billion in 2019, had expanded to KRW 18.7 billion in 2020, KRW 25.5 billion in 2021, KRW 33.5 billion in 2022, and KRW 40 billion last year. This year, the market reached KRW 27.5 billion in the first half of the year alone and is expected to exceed KRW 50 billion by the end of the year.
- Company
- Minjung Jung to head Corporate Affairs at Sanofi KR, NZ, AU
- by Eo, Yun-Ho Sep 26, 2024 05:51am
- Sanofi’s Executive Director Minjung Jung (48) has been named head of Corporate Affairs for the Sanofi Group's 3 subsidiaries - Korea, New Zealand, and Australia. The appointment follows the appointment of Kyungeun Bae (53) as the General Manager of Pharma MCO South Korea and Australia/New Zealand & MCO Lead in the first half of last year, raising the stature of Sanofi Korea. With this promotion, Jung will now lead Corporate Affairs for the three countries, effective September 9th. The Corporate Affairs department is responsible for Public Affairs, Communications, CSR, Government, and Affairs. Jung is a seasoned public affairs professional who joined Sanofi in September last year. Since starting her career at MSD Korea in 2007, Jung has served various marketing roles at Boehringer Ingelheim Korea, Merck Korea, and Amgen Korea. Sanofi has been executing a Play To Win strategy, which focuses on building innovation platforms to develop first-in or best-in-class therapies and vaccines from 2020. The plan is to drive long-term growth through a virtuous cycle of efficiently redeploying resources to accelerate innovation and maximize R&D productivity. As a key part of this strategy, Sanofi has been operating its Korea and Australia & New Zealand subsidiaries as one integrated organization.
- Company
- "Bird flu: a new emerging pandemic risk factor"
- by Son, Hyung Min Sep 25, 2024 05:49am
- Jacob Lee, Professor of the Division of Infectious Disease at Hallym University Kangnam Sacred Heart Hospital.The industry is preparing against avian influenza, which can be transmitted from animal to human, as it is predicted to be the next pandemic risk factor. Experts suggest that an improved vaccine technology development and manufacturing system are required ahead of the emerging pandemic following COVID-19. CSL Seqirus Korea held a press conference on September 24th at Hotel President in Seoul to share the recent advances in avian influenza. During the meeting, Jacob Lee, Professor of the Division of Infectious Disease at Hallym University Kangnam Sacred Heart Hospital, emphasized strengthening Korea's response measure strategy for avian influenza. Avian influenza is an infectious respiratory disease that commonly occurs in wild birds. Recently, there have been cases of animal and human infections in addition to poultry and wild birds. In March, a death case was reported in Vietnam related to avian influenza human infection. The highly pathogenic H5N1 strain of avian flu, which emerged from a type of influenza A and is spreading world-wide, has infected over 300 species of birds and over 40 species of animals. 14 cases related to H5N1 human infection transmitted from cows and birds were reported in the United States alone. Several duck farms in South Korea have recently reported H5N1 infection cases. The Korea Disease Control and Prevention Agency (KDCA) is preparing responses to potential influenza pandemic, such as holding a symposium to discuss response plans. Lee said, "Although continual human-to-human infection has not been reported, there are increasing cases of animal-to-human infection. The academics consider avian influenza as the risk factor for causing the next pandemic." And added, "We could use vaccine technologies developed for COVID-19, such as the messenger RNA (mRNA) platform, for influenza. Establishing these platforms can reduce development time once an antigen is chosen." mRNA vaccines are composed of mRNA molecules and lipid nanoparticles surrounding it. mRNA contains a genetic material that can synthesize target proteins, and lipid nanoparticles protect mRNA, working as a transporter to deliver mRNA into the body. Recent studies showed that lipid nanoparticles can function as not only an mRNA transporter but also an 'immune enhancer,' increasing immune responses to vaccines. Lee says that Korea needs to secure vaccines that can enhance immunity since technology is still lacking. CSL Seqirus is known to manufacture vaccines rapidly through its platform. The company can readily shift from manufacturing seasonal vaccine influenza to pandemic influenza vaccine using its global manufacturing network. Moreover, the company manufactures and supplies vaccines for viral variations and zoonotic viruses. Marc Lacey, CSL Seqirus Global Pandemic Head, said, "We have built vaccine technologies through years of vaccine development experiences. For example, MF59 can enhance immune responses with a small antigen dosage. For H5N1 avian influenza variant, it only took 79 days until the vaccine developed. Our company can readily respond to global pandemic influenza." Lee said, "Vaccines that can be produced domestically in South Korea are egg-based and cell-based. Korea needs to develop the mRNA vaccine platform and immune enhancers. If the development is difficult, we must establish a system to introduce vaccines from overseas."
- Company
- ‘Link the processes to improve access to orphan drugs'
- by Kim, Jin-Gu Sep 25, 2024 05:49am
- A claim has been raised that the approval, evaluation, and negotiation linkage system should be introduced to strengthen access to rare disease drugs. Also, the claim that the pharmacoeconomic evaluation system should be flexibly applied to rare disease drugs and that the scope of the risk-sharing agreement system be expanded was raised at the time. Seung-Rae Yu, a professor at Dongduk Women's University College of Pharmacy, made the arguments above at the ‘Roundtable on Improving Access to Rare Disease Drugs' that was hosted by NA Representative Youngseok Seo (NA Welfare and Health Committee) that was held on the 24th. While introducing a study that was conducted on rare disease patients in Korea, Professor Yu said that 8 out of 10 patients feel a financial burden in the process of treating rare diseases without reimbursement. He pointed out that even though the government has continuously strengthened coverage, the number of cases for which treatments were not introduced to Korea or had not been listed for reimbursement in the long term has been increasing. In response, Yu emphasized the need to introduce a system that combines approval, evaluation, and negotiation to further speed up the reimbursement coverage process in Korea. In this regard, the government is currently conducting a pilot project that conducts reimbursement evaluations at the same time as the marketing authorization application. Yu argued that the pilot project should be expanded into a full-scale project. He also stressed the need to flexibly apply the pharmacoeconomic evaluation to rare disease treatments. He said, “The ICER threshold for the pharmacoeconomic evaluation should be flexibly applied to diseases that irreversibly worsen patients’ lives and cause disease burden, and the application of the risk-sharing system should be expanded to improve Korea’s reimbursement rate.” Yu added that priorities for strengthening coverage should be determined through public discussion in the mid-to-long term. “Given the status of new drugs introduced in Korea compared to major countries and the disparity in the proportion of new drug expenditure within the total drug expenditure, we need to prioritize reimbursement for diseases that have a high disease burden.” The government gave a generally positive evaluation to the approval-evaluation-negotiation linkage pilot project. However, the government announced plans to address the issues identified in the 1st pilot project and proceed with the 2nd pilot project. Eun-Joo Lee, an official from the Ministry of Health and Welfare’s Division of Pharmaceutical Benefits, explained, “The pilot project linking the approval-evaluation-negotiation system is underway. Two drugs were selected for the first pilot project, and we are now preparing to launch the 2nd pilot project.” Lee added, “We had difficulties during the first pilot project,” he said, ”In the case of one drug, it was difficult to objectively measure the drug’s cost-effectiveness due to problems with the evaluation tool in the process of deciding whether to reimburse the drug. We plan to reflect this in the second project.”
- Company
- Cancer mRNA vaccine shows potential in brain tumor
- by Son, Hyung Min Sep 24, 2024 05:46am
- Therapeutic cancer vaccines have been showing promise in central nervous system diseases. CureVac recently confirmed the safety of its messenger ribonucleic acid (mRNA) vaccine, CVGBM, in glioblastoma in a Phase I clinical trial. Immunomic Therapeutics and IM Biologics have also taken up the challenge of developing cancer vaccines for brain tumors. Cancer vaccines are therapeutic vaccines that treat cancer by administering cancer cell antigens to patients and activating the immune system rather than prevention vaccines. Like conventional vaccines, cancer vaccines also work with the same mechanism of action, activating the body’s immune response and inducing immunogenic cell death. Major pharmaceutical and biotech companies are focusing on maximizing the treatment effect rather than prevention, and are using strategies to increase their vaccine’s commercialization potential by combining its use with anticancer drugs. Cancer vaccines with various mechanisms of action are being developed...targets brain tumors According to industry sources on the 23rd, Germany's CureVac recently unveiled the first-in-human trial results of its mRNA cancer vaccine candidate 'CVGBM' in treating glioblastoma. Glioblastoma is a type of glioma, a malignant tumor that occurs primarily in the brain. Glioblastoma has a poor prognosis, with a 5-year survival rate of a mere 5% despite surgery and chemoradiotherapy. The average survival time is only about one year. CureVac’s CVGBM is an investigational therapeutic mRNA-based vaccine encoding 8 epitopes derived from tumor-associated antigens with potential relevance in glioblastoma. An mRNA cancer vaccine constructs abnormal proteins (neoantigens) produced by cancer cells. The body's immune cells then produce antibodies to respond to them, which then kills cancer cells. CureVac is currently conducting a Phase I trial in MGMT-unmethylated glioblastoma, which is known to have a poor prognosis. As of February 29, 2024, 16 patients with glioblastoma have been enrolled in the trial. The primary endpoints were safety, tolerability, and dose-limiting toxicities (DLTs) in CVGBM. Immunogenicity was included as an exploratory endpoint. Clinical results showed that the most common treatment-emergent adverse events (TEAEs) were chills (13 patients), fever (12 patients), headache (12 patients), and fatigue (11 patients). Most patients experienced mild-to-moderate symptoms. “CVGBM was generally well tolerated with an acceptable safety profile,” the researchers concluded. ”Based on all available safety data, the dose selected for clinical expansion was 100 μg. Patients did not reach the maximum tolerated dose.” CureVac is continuing to monitor clinical progress and plans to present the first immunogenicity data once results are confirmed. Meanwhile, a U.S. company, Immunomic Therapeutics has completed a Phase II clinical trial of its glioblastoma cancer vaccine, ITI-1000. ITI-1000 is a cancer vaccine based on Immunomic's cell therapy vaccine platform (UNITE) that activates “dendritic cells,” the immune system's watchdogs, to kill tumors. ITI-1000 activates dendritic cells to recognize the ‘pp65′ protein of cytomegalovirus, which is present in glioblastoma and alerts other key immune cells when a pathogen invades the body. Currently, Immunomic has completed its Phase II clinical trial and is in the process of analyzing the data. Published clinical results to date have shown that ITI-1000’s median overall survival (OS) is over 30 months. Companies in Korea are also developing cancer vaccines targeting brain tumors. IM Biologics' glioblastoma cancer vaccine candidate 'IMB-402' was selected as a new project of the Ministry of Trade, Industry and Energy's ‘Material Parts Technology Development Project.’ IMB-402 is a cancer vaccine candidate that selectively proliferates and activates glioblastoma (GBM)-specific T cells by applying an ampeptide-HLA complex to ePENDY, an IgM antibody-based multimer platform. IM Biologics designed IMB-402 with additional co-stimulatory factors to prevent activated T cells from falling into a lethargic state. IMB-402 is currently in the candidate optimization phase, and the company plans to secure the candidate and conduct non-clinical studies through this project.
- Company
- 'Keytruda' attempts at reimbursement for TNBC
- by Whang, byung-woo Sep 24, 2024 05:46am
- The industry watches whether Keytruda (pembrolizumab), which demonstrated treatment benefits in early triple-negative breast cancer, will pass the reimbursement hurdle. As the Cancer Disease Review Committee (CDRC) of the Health Insurance Review and Assessment Service (HIRA) convenes in October, whether Keytruda will set a ground to expand 17 indications will be watched. Product photo of KeytrudaAccording to the pharmaceutical industry, the HIRA will convene the 7th Cancer Disease Review Committee (CDRC) meeting on October 2nd. Currently, Imfinzi (durvalumab) and Jemperli (dostarlimab) are applying for reimbursement expansion. The review of Keytruda, which has applied for reimbursement of 17 indications, is garnering attention. In April, the CDRC review postponed setting the criteria for Keytruda, saying, 'We will reconsider setting the reimbursement criteria when the company submits a supplement for financial contribution.' While many indications seek reimbursement listing, tiple-negative breast cancer outcome is gaining attention. MSD presented the KEYNOTE-522 study, which confirmed the overall survival (OS) data for Keytruda in high-risk early triple-negative breast cancer, during the recent European Society for Medical Oncology (ESMO) Congress 2024. The KEYNOTE-522 study is a Phase 3 clinical trial evaluating Keytruda in combination with cancer chemotherapy as a perioperative adjuvant therapy. During the median 75.1 months follow-up, Keytruda perioperative adjuvant therapy in patients with high-risk early triple-negative breast cancer significantly improved the OS compared to the placebo group. Sohn, Joo Hyuk, Professor of the Division of Oncology at Yonsei Cancer Hospital, said, "In early cancer treatment aiming for complete recovery, it is not easy to demonstrate improvement in the OS rate. Improving the OS rate is a meaningful result in that it indicates saving lives of patients with early triple-negative breast cancer." The experts suggest that since the study results demonstrate clinical data to be considered for reimbursement evaluation, it may be sufficient to prove its effectiveness. Park Yeon Hee, Professor of the Department of Hematology-Oncology at Samsung Medical Center, said, "With data demonstrating improvement in OS rate, it must be considered for reimbursement review," adding, "Because patients with triple-negative breast cancer are young and economically active, the drug's societal value must be considered as well." Park added, "For patients, a diagnosis of triple-negative breast cancer diagnosis may have felt like a disaster. Keytruda offers hope to these patients. It is meaningful that early intervention may eliminate the need for follow-up treatment." The problem will likely be the cost. Because the CDRC review in April stated, 'We will reconsider setting the reimbursement criteria when the company submits a supplement for a financial contribution,' the company may need to make more financial contribution. However, the experts suggest that reimbursement for the drug may be possible for use in combination with perioperative chemotherapy, where the volume of use at 8 cycles is predictable, when considering its impact on the National Insurance finance. As the company applies for reimbursement of Keytruda's 17 indications, approval outcome by individual indication is not easily determined. Whether Keytruda's triple-negative breast cancer indication will pass the reimbursement hurdle is not clear. An industry personnel said, "Keytruda's reimbursement application, including the individual indication with few patients, has drawn attention, but the company is discussing other strategies after not passing the CDRC review," adding, "The company will likely think over the strategy after the upcoming CDRC review now that it has more indications than the initial 13."