- LOGIN
- MemberShip
- 2025-12-20 12:51:40
- Company
- Aftermath of Januvia's price cut…MSD-CKD compensation fight
- by Son, Hyung Min Jul 14, 2025 06:04am
- Chon Kun Dang Regarding transaction for price difference compensation following the drug price reduction for the diabetes treatment Januvia, Chong Kun Dang and MSD Korea have not reached agreement during negotiation for nearly two years. While the distribution industry continues to demand compensation for losses incurred due to the drug price cut, the lack of clarity on who is responsible for the settlement is exacerbating confusion and burden in the field. According to industry sources on July 14, Chong Kun Dang recently sent an official letter to the Korea Pharmaceutical Distributors Association (KPDA), officially announcing its stance on the distribution industry's request for price difference compensation related to the drug price reduction of 'Januvia Family' products, including Januvia·Janumet. The distribution industry has been requesting price difference compensation from pharmaceutical companies for price differences on inventory since the sequential drug price reductions of Januvia (sitagliptin) on September 2, 2023, Janumet XR (sitagliptin·metformin) on the same date, and Janumet (sitagliptin·metformin) on October 1, 2023. The maximum price drops by dosage for each product indicate that Januvia 100mg was reduced from KRW 846 to KRW 592, Janumet XR 100/1000 mg from KRW 831 to KRW 572, and Janumet 50/1000 mg from KRW 520 to KRW 420. The maximum price difference per tablet amounted to KRW 259. However, as no compensation has been made to date, complains are growing within the industry. In response, Chong Kun Dang recently sent an official letter to the KPDA, stating that discussions regarding the responsible party have not yet concluded. According to the official letter, Chong Kun Dang acknowledges that the drug price reductions for Januvia and Janumet, due to patent expirations, were implemented sequentially between September and October 2023, and that distributors have been consistently requesting compensation. Chong Kun Dang explained that the reason no proper compensation has been made yet is a complex situation where Chong Kun Dang demanded price difference compensation from MSD Korea, the marketing authorization holder (MAH) at the time of the drug price reduction, but MSD Korea refused. Januvia is a DPP-4 inhibitor, containing sitagliptin, used to treat diabetes and is developed by MSD. Following Januvia's launch, MSD and Chong Kun Dang have been jointly promoting these products since 2016. However, in 2023, MSD Korea reorganized its chronic disease business unit to focus on its oncology and vaccine businesses, transferring the rights for the Januvia Family to Chong Kun Dang. Chong Kun Dang has been exclusively handling the domestic sales of Januvia as of July 15, 2023. MSD Korea's position is that since the marketing and revenue rights were already transferred at that time, they bear no responsibility for compensation related to the drug price reduction after September 2023, when the price cut occurred. An MSD Korea official stated, "We transferred all domestic rights, including marketing and manufacturing rights, for Januvia products to Chong Kun Dang in July 2023, transferring exclusive sales and marketing authority," and added, "Accordingly, Chong Kun Dang is responsible for price difference compensation due to drug price reductions that occurred after that point." The official added, "We are willing to compensate for inventory that we sold before July 2023, before Chong Kun Dang acquired the marketing rights." The official further explained, "It is true that the transfer of MAH was delayed until July 2024, but this was because time was needed for actual transfer preparations, such as changing product labels," and added, "At the time of the drug price reduction in September 2023, we were not engaged in actual revenue-generating activities, and all related rights had been transferred to Chong Kun Dang. The company was only the MAH on paper." Negotiations ongoing due to discrepancy between marketing rights and MAH...Distribution industry "we're troubled" The key issue is related to the division of roles and accountability between the MAH and the actual operating entity at the time of the drug price reduction. MSD Korea maintains that since it transferred domestic marketing and revenue rights for Januvia to Chong Kun Dang as of July 2023, Chong Kun Dang is responsible for price difference compensation arising from subsequent drug price reductions. Conversely, Chong Kun Dang stresses that MSD Korea still held the marketing authorization at the time of the drug price reduction. Januvia's marketing authorization was indeed transferred to Chong Kun Dang on July 23, 2024, after which the business transitioned to a global direct import structure. Chong Kun Dang believes that "Shifting the responsibility for compensation without the official change of the marketing authorization holder is unfair." However, given the sensitivity of the issue, they are taking a reserved stance by stating that it's difficult to issue an official statement and that discussions with MSD Korea are still ongoing. In 2016, MSD Korea and Chong Kun Dang have signed an agreement to jointly promote treatments for diabetes and dyslipidemia, including Januvia. However, as negotiations delay, the burden of price difference compensation due to the drug price reduction is ultimately being passed on to pharmaceutical distributors. The pharmaceutical distribution industry has consistently demanded compensation for the drug price difference of Januvia family products since the second half of 2023. However, settlement has been delayed due to the ambiguity of accountability between the two companies. The recent official letter sent by Chong Kun Dang to the KPDA directly mentioned this dissatisfaction from the distribution industry. The letter stated, "Discussions between the two companies are ongoing, but as the compensation discussion has not been finalized, distributors are facing difficulties," adding, "We will proactively work towards a swift resolution with responsibility." An official from a pharmaceutical distribution company pointed out, "The pharmaceutical companies that should be responsible for settling the price difference due to the government's drug price reduction are pushing responsibility onto each other," and added, "Distributors compensate pharmacies for the price difference but receive no compensation from the pharmaceutical companies, thus bearing the entire burden in the middle." The industry widely expects the transfer of Janumet XR's marketing authorization in November, raising concerns that the same problem could recur. If the discussion over responsibility drags on while the drug prices of major sitagliptin-based diabetes treatments like Januvia, Janumet, and Janumet XR are sequentially reduced. In that case, distributors will inevitably continue to bear the settlement burden. Ultimately, this discussion goes beyond individual products and extends to the industry-wide challenge of how to resolve systemic blind spots that can arise from the separate structures of marketing rights and marketing authorization.
- Company
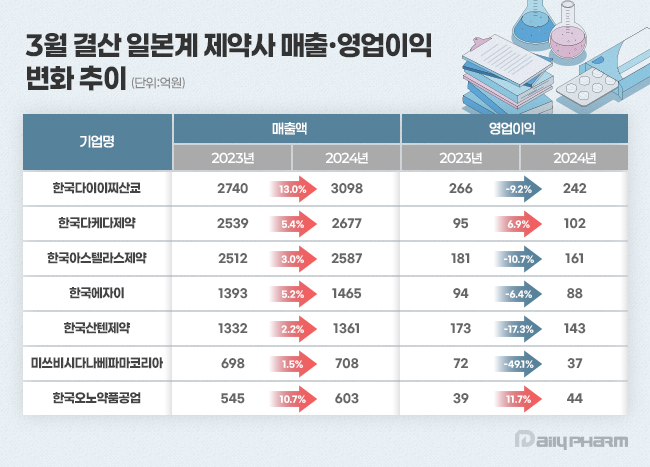
- Daiichi Sankyo and Ono Pharmaceutical see surge in sales
- by Son, Hyung Min Jul 14, 2025 06:03am
- Japanese pharmaceutical companies operating in South Korea saw overall sales growth last year. All seven companies surveyed achieved year-on-year sales growth, with Daiichi Sankyo Korea and Ono Pharma Korea leading the way with double-digit growth rates. However, in terms of operating profits, the companies showed mixed emotions. According to the Financial Supervisory Service's electronic disclosure system on the 11th, the performance of 7 Japanese pharmaceutical companies with March fiscal year-ends totaled at KRW 1.2499 trillion, up 6.3% from KRW 1.1759 trillion the previous year. During the same period, operating profit decreased by 11.2% from KRW 92 billion to KRW 81.7 billion. Major Japanese pharmaceutical companies' Korean subsidiaries follow the Japanese fiscal year, recognizing the period from April last year to March this year as their 2024 annual sales. #SB Daiichi’s sales surpass KRW 300 billion for the first time... Ono’s sales double in four years Last year, Daiichi Sankyo Korea's sales increased by 13.0% year-on-year to KRW 309.8 billion. During the same period, operating profit decreased by 9.2% from KRW 26.6 billion to KRW 24.2 billion. Daiichi Sankyo Korea's sales have been steadily increasing since 2020. The company surpassed KRW 200 billion in sales for the first time in 2020 with KRW 217.9 billion, followed by KRW 245.4 billion in 2021, KRW 253.2 billion in 2022, and KRW 274 billion in 2023, continuing its upward trend. In particular, it is analyzed that the company achieved synergistic effects by collaborating with the domestic pharmaceutical company Daewoong Pharmaceutical on certain cardiovascular products such as Lixiana and Sevikar. Daiichi Sankyo Korea signed a co-marketing agreement with Daewoong Pharmaceutical for Sevikar in 2013 and Lixiana in 2015, and has maintained its partnership with the company to date. Among these, the highest-selling product is the direct-acting oral anticoagulant (DOAC) Lixiana. According to market research firm UBIST, Lixiana's prescription sales last year reached KRW 117.5 billion, an 11.6% increase from KRW 105.3 billion in 2023. The three-component hypertension combination drug Sevikar HCT also maintained its growth momentum. Last year, Sevikar HCT's prescription sales reached KRW 42.1 billion, up 4.0% from the previous year. Daiichi Sankyo Korea generated approximately KRW 140 billion in prescription sales from olmesartan-based hypertension treatments, including Sevikar HCT (KRW 42.1 billion), Olmetec (KRW 30.6 billion), and Sevikar (KRW 68.8 billion). Daiichi Sankyo Korea is working to expand its business from cardiovascular drugs to anticancer drugs. In particular, the company is focusing on new growth areas such as antibody-drug conjugates (ADCs) by concentrating its R&D capabilities. Following the approval of Enhertu, the company is preparing to launch various treatments, including five ADC strategies such as Datroway, Patritumab deruxtecan, DS-7300, and DS-6000. Last year, Takeda Pharmaceutical Korea's sales increased by 5.4% year-on-year to KRW 267.7 billion. Operating profit rose by 6.9% to KRW 10.2 billion. Anticancer drugs such as Zejula, Alunbrig, and Ninlaro contributed to the sales growth through steady growth. According to the market research firm UBIST, Alunbrig recorded prescription sales of KRW 12.1 billion last year. Since its launch in 2019, its prescription sales have continued to increase. This treatment is gaining market share for its indication in ALK-positive non-small cell lung cancer. Alunbrig is in fierce competition with Pfizer's Lorviqua and Roche's Alecensa. However, However, Alunbrig offers the advantage of once-daily, single-tablet dosing, and can be taken regardless of food intake. This simplicity reduces the number of factors patients need to consider when taking the medication. The ovarian cancer treatment Zejula also recorded KRW 14.3 billion in sales last year, a 51.6% increase in prescriptions from the previous year. In October last year, Zejula was added to the health insurance reimbursement list for the treatment of HRd-positive ovarian cancer. Until then, Zejula had been reimbursed only as maintenance therapy for BRCA-mutated ovarian cancer patients who responded to platinum-based therapy as a first-line treatment for ovarian cancer. The growth of Ono Pharma Korea was also noteworthy. Ono’s sales last year were KRW 60.3 billion, up 10.7% year-on-year. Operating profit in the same period increased 11.7% from KRW 3.9 billion to KRW 4.4 billion. The sales and operating profit recorded by Ono last year are the highest figures since the company began submitting audit reports in 2017. Ono recorded sales of over KRW 40 billion in 2021 and surpassed KRW 50 billion for the first time the following year. Comparing the KRW 60.3 billion recorded by Ono last year with the KRW 31 billion in sales in 2020, sales increased by 94.5% over four years. The immune-oncology drug Opdivo drove Ono’s sales growth. Opdivo is an anti-PD-1 class immuno-oncology drug jointly developed by Ono and BMS, and was approved in Korea in 2015. Ono holds development and sales rights in Asia, including South Korea, Japan, and Taiwan. In particular, the combination of Opdivo and BMS's CTLA-4 targeted immunotherapy Yervoy has demonstrated efficacy in various solid tumors such as melanoma, renal cell carcinoma, and hepatocellular carcinoma, contributing to the increase in market share. According to market research firm IQVIA, Opdivo surpassed KRW 100 billion in domestic sales in 2022. All showed external growth, but with mixed operating profits Astellas Pharma Korea, Eisai Korea, and Santen Pharmaceutical Korea all saw slight increases in sales, but their operating profits all declined. Astellas Pharma Korea reported sales of KRW 258.7 billion last year, a 3.0% increase from the previous year. Harnal, Betmiga, Prograf, and Xtandi performed well, driving sales growth. Harunal, a treatment for benign prostatic hyperplasia, recorded prescription sales of KRW 66.2 billion last year, a 0.8% increase from the previous year. Betmiga, a treatment for overactive bladder, and Prograf, an immunosuppressant, have consistently recorded prescription sales of over KRW 30 billion even after their patents expired. Last year, prescription sales for Betmiga and Prograf were KRW 33.4 billion and KRW 33.6 billion, respectively, up 2.6% and 3.9% year-over-year. Operating profit decreased by 10.7% year-on-year to KRW 16.1 billion. A slight increase in the cost of sales contributed to the decrease in gross profit. Astellas Pharma Korea is pinning its hopes on its next-generation anticancer drugs. The company's Padcev, its ADC anticancer drug, and Vyloy, its new gastric cancer targeted therapy targeting Claudin 18.2, have entered the domestic market. Both drugs have shown remarkable results in clinical trials. Currently, Astellas Pharma Korea has completed its application for insurance reimbursement for both drugs. Eisai Korea's sales rose 5.2% from KRW 139.3 billion in 2023 to KRW 146.5 billion last year. Operating profit for the same period decreased 6.4% to KRW 8.8 billion. Sales of existing products such as the anticancer drug Lenvima, the gastroesophageal reflux disease treatment Pariet, and the Alzheimer's disease treatment Aricept remain steady. The three products—Aricept (KRW 95.9 billion), Pariet (KRW 17.9 billion), and Lenvima (KRW 12.9 billion)—combined for over KRW 100 billion in sales last year. Operating profit decreased slightly due to factors such as increased cost of sales. EisaiEisai is expecting strong performance from its new Alzheimer's disease drug, lecanemab. Lecanemab is an Alzheimer's disease treatment developed by Eisai and Biogen that selectively binds to amyloid beta, a substance believed to cause the disease, and has been proven to slow the progression of the disease and delay cognitive decline. It is reported that lecanemab is currently being prescribed at various general hospitals and semi-general hospitals in Korea. Santen Korea reported sales of KRW 136.1 billion last year, a 2.2% increase from the previous year. However, operating profit decreased by 17.3% to KRW 14.3 billion. Santen specializes in ophthalmic products and holds various formulations, including diquafosol. However, in South Korea, hyaluronic acid eye drops are primarily used, and the company has not significantly increased its prescription volume in Korea. As of last year, the product with the highest sales among Santen Pharmaceutical's portfolio was Cosopt S eye drops, used for glaucoma. Cosopt S’s prescription volume last year was KRW 33.9 billion, an increase of 2.6% compared to the previous year. Mitsubishi Tanabe Pharma Korea's sales last year were KRW 70.8 billion, up 1.5% from 2023, but operating profit fell 49.1% in the same period. The decrease in operating profit was due to an increase in selling, general, and administrative expenses, such as reimbursement and retirement benefits, as well as an increase in cost of sales.
- Company
- Bimzelx may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jul 11, 2025 06:12am
- The new psoriasis drug Bimzelx may be prescribed at general hospitals in Korea. According to industry sources, UCB Pharma Korea's Bimzelx (bimekizumab) has been approved by the Drug Committees (DCs) of major hospitals nationwide, including tertiary hospitals like Seoul Asan Medical Center and Severance Hospital as well as major hospitals including Kyungpook National University Hospital, Pusan National University Hospital, Seoul National University Bundang Hospital, Chonnam National University Hospital, Jeonbuk National University Hospital, and Hanyang University Hospital. The company appears to be rapidly working to expand Bimzelx prescriptions following its reimbursement listing in June. i1Bimzelx is the first plaque psoriasis treatment that dually inhibits interleukin-17A and 17F (IL-17A and 17F). IL-17A and IL-17F are key cytokines that trigger the inflammatory process in psoriasis, and Bimzelx selectively and directly targets and inhibits both simultaneously. This dual inhibition mechanism against IL-17A and 17F is what makes the drug’s introduction significant. Despite the emergence of various psoriasis treatments, there remained an unmet need in practice due to resistance and other factors, which is why health professionals saw the introduction and reimbursement of Bimzelx encouraging. Yong-Beom Choi, President of the Korean Society for Psoriasis (Department of Dermatology, Konkuk University Medical Center), said, “Psoriasis is an intractable disease that recurs and improves repeatedly, and the quality of life of patients with severe psoriasis in particular tends to deteriorate significantly, affecting their mental health. Therefore, the reimbursement and launch of Bimzelx, which has been proven to be highly effective, is very meaningful for both medical professionals and patients.” He added, “Based on its next-generation mechanism of action, Bimzelx is expected to be an excellent treatment option for both new patients and those who have not seen sufficient results with existing treatments.” Meanwhile, in the Phase III BE READY trial, 90.8% of patients in the Bimzelx group achieved PASI 90 at Week 16, and 68.2% of patients achieved PASI 100. In another clinical trial that compared Bimzelx with another biological agent, there was a clear difference in the percentage of patients who achieved complete clearance of skin lesions at Week 16, or 'PASI 100'. Specifically, PASI 100 for the drug and control drug were as follows: ▲BE VIVID: Bimzelx 59%, ustekinumab (Stelara) 21% ▲BE SURE: Bimzelx 60%. 8%, adalimumab (Humira) 23.9% ▲BE RADIANT: Bimzelx 61.7%, secukinumab (Cosentyx) 48.9%, etc.
- Company
- Gastric cancer-targeted therapy 'Vyloy' seeks reimb again
- by Eo, Yun-Ho Jul 10, 2025 06:10am
- Product photo of Vyloy Vyloy, a gastric cancer-targeted therapy, is once again vying for inclusion in the National Health Insurance reimbursement list. According to industry sources, Astellas Pharma Korea recently submitted a reimbursement application for Vyloy (zolbetuximab), a targeted treatment for Claudin 18.2-positive gastric cancer. Vyloy did not pass the Health Insurance Review & Assessment Service's (HIRA) Cancer Disease Review Committee (CDRC) in February. Consequently, it remains to be seen whether it will achieve success in this second attempt. Vyloy, approved in Korea last September, is the world's first approved Claudin 18.2-targeted therapy. It is an immunoglobulin monoclonal antibody that acts by binding to Claudin 18.2, a protein expressed and exposed in the stomach. The basis of Vyloy's approval was the SPOTLIGHT Phase 3 study, which showed that the median progression-free survival (mPFS) for the Vyloy and mFOLFOX6 (oxaliplatin, leucovorin, fluorouracil) combination therapy was 10.61 months, higher than the placebo group's 8.67 months. The median overall survival (mOS) for the treatment group also surpassed that of the placebo group, which was 15.54 months, reaching 18.23 months. The GLOW study also found that the Vyloy and CAPOX (capecitabine and oxaliplatin) combination therapy recorded a median PFS of 8.21 months, reducing the risk of disease progression or death by approximately 31%. However, Vyloy is currently an unreimbursed drug. It was submitted to HIRA's CDDC in February but failed to establish reimbursement criteria. Additionally, due to a companion diagnostic (CDx) issue last year, Vyloy was officially launched in Korea only in March. To use Vyloy, it's necessary to identify patients who are Claudin 18.2-positive. This was complicated because the companion diagnostic device used for Claudin 18.2 diagnosis was being considered for the evaluation of new medical technology. In response, Astellas initiated an Expanded Access Program (EAP) even before Vyloy's approval to ensure that patients in need could access the treatment quickly. Currently, 51 patients are enrolled in 10 institutions through this program. Professor Sun Young Rha of Yonsei Cancer Center's Division of Medical Oncology stated, "Approximately 90% of metastatic gastric cancer patients are HER2-negative, creating a critical need for new biomarker-targeted therapies." Rha added, "In a situation where about 40% of HER2-negative patients are reported to be Claudin 18.2-positive, the introduction of Vyloy, which selectively binds to Claudin 18.2, presents new treatment possibilities."
- Company
- Ono Pharma Korea’s sales double in 4 years with Opdivo
- by Son, Hyung Min Jul 10, 2025 06:09am
- Ono Pharmaceutical Korea continued its sales growth, powered by its immuno-oncology drug Opdivo. The company's sales doubled in 4 years. The company is preparing for the expiration of Opdivo's patent by securing a diverse pipeline of new anticancer drugs, including antibody-drug conjugates (ADCs) and novel immuno-oncology drugs with different mechanisms of action. According to the Financial Supervisory Service's electronic disclosure system on the 9th, Ono Pharmaceutical's sales last year reached KRW 60.3 billion, a YoY increase of 10.7%. During the same period, operating profit rose by 11.7% from KRW 3.9 billion to KRW 4.4 billion. The sales and operating profit recorded by Ono Pharmaceutical last year are the highest figures since the company began submitting audit reports in 2017. Ono Pharmaceutical recorded sales of over KRW 40 billion in 2021 and exceeded KRW 50 billion for the first time the following year. Comparing the company’s sales of KRW 60.3 billion last year with the KRW 31 billion in 2020, it has increased by 94.5% in four years. The immuno-oncology drug Opdivo drove Ono Pharmaceutical's sales growth. Opdivo is an anti-PD-1 class immune-oncology drug jointly developed by Ono Pharmaceutical and BMS, and was approved in Korea in 2015. Ono Pharmaceutical holds the development and marketing rights for Opdivo in Asia, including Korea, Japan, and Taiwan. Ono Pharmaceutical's sales have risen significantly since 2017, when Opdivo was granted reimbursement. The first indication reimbursed was for non-small cell lung cancer. Ono Pharmaceutical's sales in 2018 were KRW 44.8 billion, up 44.4% from KRW 31 billion the previous year. However, competition from MSD's Keytruda had an impact on Opdivo’s sales. Opdivo recorded sales of KRW 32.4 billion in 2019, down 27.8% from the previous year. Ono Pharmaceutical sought to reverse the trend by expanding the indications and reimbursement for Opdivo. In particular, the combination of Opdivo and BMS's CTLA-4 targeted immuno-oncology drug Yervoy has been effective in various solid cancers, such as melanoma, renal cell carcinoma, and hepatocellular carcinoma, contributing to an increase in market share. In addition, Opdivo became the first immuno-oncology drug to be approved for the treatment of gastric cancer in 2021. According to the market research institution IQVIA, Opdivo surpassed KRW 100 billion in domestic sales in 2022. Company busy securing future pipeline following Opdivo Ono PharmaceuticalOno Pharmaceutical is preparing for the expiration of Opdivo's patent. Opdivo's patent is scheduled to expire in the United States in 2028, followed by other markets around the world. First, the company is defending the market with Opdivo Qvantig, a subcutaneous injection (SC) formulation of Opdivo. Opdivo Qvantig was approved in the United States last year, and Ono Pharmaceutical and its partner BMS are seeking approval in major countries. The existing intravenous (IV) formulation of the anticancer drug requires administration for over an hour, but the SC formulation has the advantage of reducing the administration time to less than 10 minutes. The SC formulation of the anticancer drug can provide convenience to patients who must visit the hospital once every 3 weeks on average to receive the IV formulation. Ono Pharmaceutical is also seeking to enter the antibody-drug conjugate (ADC) market. Last year, Ono Pharmaceutical acquired LCB97, an ADC candidate substance developed by LigaChem Biosciences, a domestic ADC developer. LCB97 targets L1CAM, a protein expressed in various solid tumors, such as lung cancer, pancreatic cancer, and colorectal cancer. LCB97 incorporates LigaChem Biosciences’ proprietary ConjuALL linker technology. ADCs consist of a linker, payload (drug), and antibody, and the ConjuALL linker is evaluated to overcome issues such as the release of cytotoxic drugs in the bloodstream and attacks on normal cells. LigaChem Biosciences and Ono Pharmaceutical have also signed a contract to transfer the company’s proprietary technology for ADCs that target multiple targets along with LCB97. Under this contract, Ono Pharmaceutical has secured the rights to discover and develop ADC candidates targeting multiple targets using LigaChem Biosciences’ platform technology. Additionally, Ono Pharmaceutical has entered into a technology transfer agreement with domestic company NEX-I and will begin developing the immunotherapy candidate ‘NXI-101.’ NXI-101 is a next-generation immunotherapy drug candidate that inhibits the function of ONCOKINE-1, a newly identified target discovered through the ‘ONCOKINE’ platform, which identifies factors causing resistance to cancer immunotherapy. Currently, Ono Pharmaceutical has completed preclinical trials for NXI-101 and is conducting Phase I clinical trials.
- Company
- Credit ratings for Samsung Biologics·JW Holdings↑
- by Kim, Jin-Gu Jul 10, 2025 06:08am
- Major pharmaceutical and biotech companies (biopharma companies) are facing varying differing credit ratings and outlooks. While Samsung Biologics and JW Holdings received upward credit ratings, Handok was downgraded. Dong-A ST's credit rating outlook shifted from 'stable' to 'negative'. Credit rating agencies explained that these results to differences in individual company performance and profitability. They anticipated that the disparities among companies will become even more pronounced in the second half of the year. Credit rating adjusted upward for Samsung Biologics and JW Holdings... attributed by improved performance & stable profitability According to industry sources on the July 8, Korea Ratings·Korea Investors Service (KIS) recently upgraded Samsung Biologics' credit rating and outlook from 'AA- positive' to 'AA stable'. Korea Ratings also raised JW Holdings' rating by one grade, from 'BBB- positive' to 'BBB stable'. Samsung Biologics' stable profitability and expanding global orders were reflected in its evaluation, while JW Holdings' recovery in pharmaceutical business profitability and improved financial structure were reflected. In contrast, credit ratings·outlooks for Handok and Dong-A ST were adjusted downward. KIS adjusted Handok's credit rating downward by one grade from 'BBB+' to 'BBB', while converting its outlook from 'negative' to 'stable'. Sluggish performance and increased financial burden were cited as the primary reasons for the credit rating downgrade. Credit rating for Dong-A ST was maintained, but the company's outlook was downgraded. NICE Investors Service kept Dong-A ST's credit rating at 'A+' but adjusted its outlook from 'stable' to 'negative'. Korea Ratings also changed Dong-A ST's outlook to negative. This is analyzed as a result of a complex interplay of factors, including slowing profitability and the burden of research and development (R&D) costs. Other major biopharma companies maintained their existing credit ratings and outlooks. According to Korea Ratings, Chong Kun Dang's credit rating and outlook remained 'AA- stable' as of the end of the first half, identical to the end of last year. Chong Kun Dang Holdings, Green Cross, and Daewoong Pharmaceutical maintained 'A+ stable', while Dong-A Socio Holdings, HK inno.N, and Boryung held 'A stable'. ISU Abxis remained at 'BB- stable', and Korea Union Pharmaceutical stayed at 'CC negative'. KIS and NICE Investors Service also maintained the credit ratings and outlooks of Green Cross Holdings·Chong Kun Dang Holdings·Chong Kun Dang·HK inno.N·Dong-A Socio Holdings·SK Plasma·ISU Abxis·Vivacell Biotechnology at last year's levels. Upward·downward credit rating adjustments·outlook by companies..."It will become more polarized in the second half of the year" Overall, while the fluctuation in credit ratings·outlooks for biopharma companies was not significant, individual companies experienced mixed fortunes depending on their business structure and financial response capabilities. Combined Sales and R&D Spending Trends by Year-High R&D expenditure burden puts pressure on profitability (legend: sales (left), R&D ratio (red line, %), operating profit (blue line, %) Credit rating agencies assessed that while companies successful in external growth and profitability improvement received upward credit ratings, some pharmaceutical companies highly dependent on the domestic market are accumulating factors that burden their credit rating. Korea Ratings assessed, "In the first half, strong exports and new product launches drove overall external growth in the biopharma industry, but profitability improvement was limited due to increasing R&D costs," and added, "By companies, clear performance differentiation is observed based on the presence of high-margin products, the extent of market share secured, the scale of new business investments, and the ability to control R&D costs." This trend is expected to continue into the second half of the year. Notably, new drug development and overseas expansion are projected to have a significant impact on corporate performance. An analysis suggests that the disparity between companies could widen further, depending on the expansion of export proportions, new drug development achievements, and the pursuit of new business opportunities. In particular, the growth momentum in the biopharma sector, including Contract Development and Manufacturing Organization (CDMO), is expected to continue. Korea Ratings predicted, "New drug development, biosimilar growth, and the increasing outsourcing demand from global pharmaceutical companies will drive the growth of the CDMO industry." Combined Borrowings and Coverage Trends-Increased capital requirements expected to drive up borrowings (Legend: Net Borrowings (dark blue, Unit: KRW 100 million), Net Borrowings/EBITDA (orange line, %), Based on Combined performance of 7 companies with our credit ratings, Source: Industry data compilation, estimate from Korea Ratings) Key variables for the second half of the year were identified as 'control over financial stability and the U.S. pharmaceutical trade policy.' The explanation was provided that "the performance gap could widen further depending on how stably companies control their profitability and financial structure" in a situation where R&D burdens are expanding and the business environment is rapidly changing. Concerns also arise that "if the competitiveness of flagship products weakens or investment recovery is delayed, credit rating burden could increase due to deteriorating cash flow." Furthermore, if the U.S. applies tariffs to imported pharmaceuticals, a negative impact on the exports of generics and biosimilars by domestic biopharma companies is anticipated. In response, credit rating agencies advised, "Each company should strengthen its product portfolio and manufacturing competitiveness to review its response strategies."
- Company
- 'Ebglyss' can be prescribed at general hospitals
- by Eo, Yun-Ho Jul 09, 2025 06:10am
- Product photo of Ebglyss'Ebglyss,' a new drug for the treatment of atopic dermatitis, is now available for prescription at general hospitals. According to industry sources, Lily Korea's interleukin (IL)-13 inhibitor 'Ebglyss (lebrikizumab)' has passed the drug committees (DC) of tertiary general hospitals, including Seoul National University Hospital, Asan Medical Center in Seoul, and Sinchon Severance Hospital, as well as medical institutes, such as Korean University Anam Hospital and Seoul National University Bundang Hospital. Several medical institutes have generated prescription codes through emergency DC. After this drug was included in the insurance reimbursement this month (July), the prescription areas of this drug have expanded quickly. Ebglyss is a new biologic that selectively blocks the cytokine Interleukin (IL)-13, a primary cause of atopic dermatitis. Ebglyss was approved last August for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents aged 12 years and older (weight over 40kg) who are not adequately controlled by topical therapies or for whom these therapies are not recommended. Existing atopic dermatitis treatments include Dupixent, which inhibits IL-4 and IL-13, JAK inhibitors like Rinvoq,and Adtralza, which targets IL-13. The introduction of Ebglyss further expands the range of treatment options. As atopic dermatitis is a chronic disease that is difficult to cure and requires long treatment periods, a wide range of therapeutic options are essential. The efficacy and safety of Ebglyss have been confirmed through Phase 3 clinical studies, including ADvocate-1, ADvocate-2, and ADhere. In ADvocate-1 and ADvocate-2, which evaluated Ebglyss monotherapy, the Ebglyss group showed Eczema Area and Severity Index (EASI)-75 rates of 58.2% and 52.1% respectively, during the induction period (weeks 0-16), representing an improvement over the placebo group (16.2% and 18.1%). EASI-90 rates for the Ebglyss groups were 38.3% and 30.7% respectively, while placebo groups remained at 9% and 9.5%. EASI is the percentage improvement in eczema severity. Additionally, after one year of maintenance therapy, the Ebglyss group's EASI-75 achievement rate at week 52 was 81.7%, and the EASI-90 rate was 66.4%. These figures were higher than those of the placebo group, at 66.4%. Ebglyss is the third biologic to enter this market. The introduction of this drug has expanded patient choices, following the launch of Sanofi's Dupixent and LEO Pharma's Adtralza. However, some experts say that despite the introduction of various treatments, there are still unmet medical needs. According to Korea's atopic dermatitis guidelines, systemic treatment is strongly recommended for patients with moderate-to-severe atopic dermatitis. However, while the proportion of moderate-to-severe atopic dermatitis patients in Korea increased from 30.9% to 39.7% between 2002 and 2019, the prescription rate of systemic immunosuppressants in this patient group remained at only 5%. Professor Min Kyung Shin of Kyung Hee University Hospital's Department of Dermatology said, "Patients with severe atopic dermatitis may show different effects or side effects from each treatment depending on their age and immune status. We consider reactions to side effects like latent tuberculosis, whether the treatment can help with comorbidities, patient preference, and clinical phenotypes when treating."
- Company
- Adding amiloride effective in resistant hypertension
- by Son, Hyung Min Jul 09, 2025 06:08am
- Sungha Park, Professor of Cardiology, Severance Hospital A new treatment option has been proposed for patients with resistant hypertension that cannot be controlled even with the existing triple combination therapy for hypertension. A regimen combining an olmesartan-based triple combination therapy with the potassium-sparing diuretic ‘amiloride’ demonstrated similar blood pressure-lowering effects to spironolactone, which was previously recommended as a fourth-line treatment, with fewer side effects. The SPARE study, led by Professor Sungha Park of the Department of Cardiology at Severance Hospital, was published in the international medical journal JAMA, attracting significant attention. During an interview with Dailypharm, Professor Park emphasized that the amiloride-based combination therapy has established clinical evidence supporting its broader use in real-world clinical settings for resistant hypertension. Difficult-to-treat ‘Resistant Hypertension’...Need for use of amiloride highlighted There are approximately 12.3 million hypertension patients in South Korea, of whom 10 -15% are classified as having “resistant hypertension,” which is defined as failing to achieve target blood pressure despite the use of three or more antihypertensive medications. Patients who fail to control their blood pressure even with up to 5 antihypertensive medications are classified as having “refractory hypertension,” accounting for less than 1% of all patients. According to National Health Insurance Service data, approximately 7.4% of hypertensive patients in South Korea are diagnosed with true resistant hypertension. Patients with true resistant hypertension have a 1.5 to 2 times higher risk of developing cardiovascular diseases compared to general hypertensive patients, making active blood pressure control crucial for managing their prognosis. Resistant hypertension is treated by adding an antihypertensive drug to the three antihypertensive drugs used to control blood pressure in general hypertensive patients: calcium channel blockers, RAS blockers, and diuretics. For patients whose blood pressure remains uncontrolled even with the four-drug combination, spironolactone is recommended in the fourth line as an additional drug. Spironolactone is a diuretic that acts as an aldosterone receptor antagonist, inhibiting sodium reabsorption to lower blood pressure. If blood pressure remains uncontrolled even then, vasodilators such as beta-blockers, alpha-blockers, minoxidil, and hydralazine may be used. Professor Park stated, “The biggest issue with spironolactone is its side effects. Although spironolactone is an aldosterone antagonist, it can block sex hormones, leading to fatigue, gynecomastia in men, and menstrual irregularities in women. The most serious concern is the high risk of hyperkalemia.” He added, “Due to concerns about such side effects, its use is restricted in elderly patients or those with impaired renal function, and compliance may be low. Although spironolactone is recommended as a fourth-line drug in various clinical guidelines, it is not widely used in practice.” Professor Park said, “While spironolactone has been recognized as a typical potassium-sparing diuretic due to its proven efficacy in reducing the risk of cardiovascular disease after heart failure or myocardial infarction, amiloride has been relatively overlooked. Amiloride is a drug that was widely used in the past, as it acts as a diuretic while also increasing potassium levels.” Amiloride demonstrates non-inferiority to spironolactone To verify this, Professor Park's research team and 14 other domestic institutions conducted a study to confirm the non-inferiority of spironolactone and amiloride. This clinical trial is the first head-to-head trial comparing the two drugs. Professor Park explained, “Guidelines recommend considering amiloride for patients with poor tolerability due to spironolactone’s side effects. However, there was no randomized controlled trial (RCT) data to support this recommendation. Against this backdrop, we decided to conduct a randomized controlled trial comparing spironolactone and amiloride.” He added, “Amiloride is a potassium-sparing diuretic that directly acts on the epithelial sodium channel (ENaC) in the distal convoluted tubule to block sodium reabsorption and preserve potassium. Unlike spironolactone, which blocks aldosterone receptors, its different mechanism of action does not cause hormone-related side effects such as fatigue, gynecomastia, or menstrual irregularities.” The study, named SPARE, targeted patients whose blood pressure remained uncontrolled despite taking existing triple antihypertensive medications. The study focused on patients whose systolic blood pressure remained at or above 140 mmHg after a 4-week introductory treatment with Sevikar HCT (active ingredients: olmesartan, amlodipine, and hydrochlorothiazide), The research team then divided the patients into two groups and compared them for 12 weeks: one group additionally received amiloride 10 mg and the other additionally received spironolactone 25 mg. After 12 weeks of treatment, the average home systolic blood pressure and the rate of achieving target blood pressure in the clinic were measured. Professor Park emphasized the importance of selecting “true resistant hypertension patients” to ensure more accurate results during this process. Professor Park explained, “We focused on patients who had uncontrolled blood pressure despite taking three or more conventional antihypertensive medications. We noted that a single combination therapy drug was effective in improving medication adherence, so we administered Sevikar HCT, a three-drug combination therapy containing olmesartan, amlodipine, and hydrochlorothiazide, at an appropriate dose tailored to each patient's condition for four weeks.” He added, “Sevikar HCT has already been proven to be an effective combination with excellent blood pressure-lowering effects and target blood pressure achievement rates among olmesartan-based triple combination drugs. It is a combination with proven clinical evidence.” The study results showed that amiloride demonstrated non-inferiority to spironolactone in terms of blood pressure-lowering effects. More specifically, the average home systolic blood pressure at week 12 compared to baseline was reduced by 14.7 mmHg in the amiloride group and 13.6 mmHg in the spironolactone group. The difference in blood pressure reduction between the two groups was -0.68 mmHg, which was not statistically significant. Additionally, spironolactone was particularly effective in individuals with elevated aldosterone levels, whereas amiloride demonstrated consistent efficacy across all patients, regardless of aldosterone to renin ratio. It is known that an abnormally activated aldosterone-to-renin ratio can lead to elevated blood pressure. Professor Park stated, “Considering drug characteristics such as medication adherence, ease of use, and side effects, amiloride could serve as an alternative to spironolactone. However, further long-term follow-up studies and expanded application in practice would be necessary.” He continued, “It is uncertain whether this study can be directly applied to Caucasians, but at least in East Asian populations such as Koreans and Japanese, amiloride can be expected to provide sufficient blood pressure-lowering effects. Although it was only a three-month study, side effects were significantly reduced. There were no cases of hyperaldosteronism, and the incidence of hyperkalemia was low. These findings also relay an important message. Professor Park noted, “While amiloride has not been actively recommended in the past, major guidelines may likely incorporate this finding in the future.” The Korean Society of Hypertension is currently revising its guidelines, and there is a high likelihood that the updated guidelines will reflect this information next year. Since the study involved domestic patients, the findings will likely be reflected. The guidelines may include recommendations to use amiloride as an option for resistant hypertension when spironolactone cannot be used. I believe it may also be reflected in other guidelines as well.”
- Company
- Novo Nordisk's injectables are in short supply
- by Nho, Byung Chul Jul 08, 2025 06:35am
- Product photo of NovoRapid injNovo Nordisk's diabetes insulin injections are experiencing a short-term shortage, causing supply difficulties in prescribing settings in Korea. The products with limited supply include NovoRapid Flexpen·Novomix Flexpen·Levemir Flexpen. Novo Nordisk sent a notice to distributors, pharmacies, hospitals, and clinics late last month, informing them of the supply limited period (April·May) and the expected resolution period (August·September). According to pharmacies, product supply has been inconsistent since around March, and despite efforts to secure stock, safety reserves are already depleted. Consequently, even if prescriptions are issued over the next two months, a substitute prescription will be unavoidable. Considering that diabetic patients are typically sensitive to medication changes, the inconvenience for doctors, pharmacists, and patients due to alternative dispensing is expected to increase significantly. One anonymous pharmacist in Yongsan-gu, Seoul, stated, "The current shortage seems to be caused by adjustments in production volume of existing products due to the explosive increase in demand for Wegovy." He asked Novo Nordisk to provide clear explanation. The basis for this speculation among medical professionals lies in the phenomenal popularity of the obesity drug Wegovy, which was launched domestically in October of last year. According to IQVIA data, Wegovy's sales of KRW 60.3 billion in Q4 of last year quickly climbing up to the top of the obesity drug market. The obesity drug market size in Q3 2024 was KRW 47.4 billion, but it surged by 97.9% to KRW 93.8 billion in just one quarter following the launch of Wegovy. In Q1 of this year, Wegovy's sales reached KRW 79.4 billion, capturing a 73.2% share of the overall obesity drug market. Wegovy, which received FDA approval in April 2023, is a GLP-1 analog containing semaglutide, confirmed to reduce HbA1c. Novo Nordisk developed Wegovy, a once-weekly obesity treatment using semaglutide, after observing weight loss effects in patients during clinical trials of GLP-1 class diabetes drug candidates. Wegovy, which received FDA approval in April 2023, is a GLP-1 analog containing semaglutide, confirmed to reduce HbA1c. Novo Nordisk developed Wegovy, a once-weekly obesity treatment using semaglutide, after observing weight loss effects in patients during clinical trials of GLP-1 class diabetes drug candidates. Regarding the overall situation, Novo Nordisk stated, "This supply shortage is due to reduced production at a filling plant caused by technical issues at some overseas manufacturing facilities and is unrelated to quality·safety." Novo Nordisk added, "We are making our utmost efforts to normalize product supply as soon as possible through close and active cooperation between the headquarters and our Korean subsidiary."
- Company
- PCV21 emerges…evidence-based vaccination policies discussed
- by Whang, byung-woo Jul 07, 2025 06:10am
- "To improve pneumococcal disease prevention in Korea, an evidence-based pneumococcal vaccination policy is essential. It's crucial to evaluate the efficacy of existing vaccines and conduct cost-effectiveness assessments for new vaccines based on domestic data." Despite the implementation of the National Immunization Program (NIP), pneumococcal disease remains a significant public health issue in South Korea. Therefore, experts emphasize the need to strengthen vaccine strategies, particularly targeting high-risk populations. Dr. Jung Yeon Heo, a professor at Ajou University HospitalDr. Jung Yeon Heo, a professor at Ajou University Hospital's Infectious Diseases Department, an expert in the field, emphasized the need for a 'dual protection strategy,' which involves the direct vaccination of high-risk adults in addition to pediatric pneumococcal vaccination. Pneumococcal infection is known to be fatal for elderly people, causing not only pneumonia but also various invasive diseases such as bacteremia and meningitis. Specifically, adults aged 65 and older face a greater risk of pneumococcal pneumonia and invasive infection. The risk of infection further increases for adults with chronic diseases compared to healthy adults of the same age. Dr. Heo explained, "Invasive Pneumococcal Disease (IPD) primarily occurs in high-risk groups, including adults aged 65 and older, immunocompromised individuals, and patients with chronic kidney disease or heart disease," and added, "Generally, the prevalence of these chronic or immunocompromised conditions increases with age, leading to a higher risk of pneumococcal infection in elderly people." Since 2013, South Korea has provided protein conjugate vaccines (PCV) for children and 23-valent polysaccharide vaccines (PPSV23) for adults (aged 65 and older) through the NIP. However, while the pediatric PCV vaccination rate is high at approximately 97%, the PPSV23 vaccination rate for adults aged 65 and older is only about 54.5%. Currently, there are concerns about the intergenerational transmission of pneumococcal bacteria, as the number of grandparents caring for grandchildren increases. Regarding this, Dr. Heo stated that indirect effects of reduced adult pneumococcal infection can be expected from pediatric vaccination, based on domestic and international cases. However, he also emphasized the importance of direct vaccination for a sufficient preventive effect. Dr. Heo said, "While indirect effects of reducing pneumococcal disease in adults can be expected from pediatric vaccination, indirect effects alone are not sufficient for adequate prevention in adults," and stressed, "In addition to pediatric vaccination, adult pneumococcal vaccination is also crucial." The distribution of serotypes also highlights the importance of prevention in elderly people. According to Dr. Heo, the most common pneumococcal serotypes in Korean adults are 3 and 19A. Despite these two serotypes being included in the currently used 13-valent pneumococcal conjugate vaccine (PCV13), they still cause infections. Dr. Heo pointed out, "This shows that even though the domestic pediatric vaccination rate is high, nearing 95%, for some serotypes, pediatric vaccination alone is not sufficient for full prevention." He added, "For certain serotypes, indirect effects alone are insufficient for adequate prevention, providing evidence that adults also need pneumococcal vaccination." Discussion of sequential·single-dose vaccination strategies..."Vaccine characteristics must be considered" However, with the emergence of newly approved pneumococcal vaccines, there is also anticipation for expanding the scope of pneumococcal disease prevention. Recently, the Korean Society of Infectious Diseases issued revised recommendations, recommending sequential vaccination with PCV15 + PPSV23 or single-dose vaccination with PCV20 for all individuals aged 6 months and older, as well as for high-risk individuals aged 19-64. Regarding sequential vaccination, Dr. Heo explained, "Vaccination is needed to enhance the immunogenicity in high-risk groups for pneumococcal disease while including as many serotypes as possible. This strategy was proposed because combining PCV's strong immune induction effect with PPSV23's broad serotype coverage can lead to more comprehensive and potent preventive effects." He also stated, "If patient convenience is prioritized, a single injection of PCV20 might be a simpler approach." However, he added, "The main reason why the sequential vaccination strategy is recommended is due to considerations of PPSV23's efficacy and cost-effectiveness." Currently, PPSV23, provided free through the domestic NIP, is considered highly cost-effective as it can prevent a wide range of serotypes at a relatively low cost. Conversely, individuals who can afford to cover the cost may opt for non-reimbursed vaccination with a single dose of PCV20, which is not covered by insurance benefits. Dr. Heo advised, "For those who can bear the cost, a single-dose PCV20 strategy can be considered. However, for those who wish to benefit from the NIP, sequential vaccination with PCV15 and PPSV23 is a good choice." He further stated, "Since each vaccine has its pros and cons and overall effects are similar, it's difficult to conclude that one vaccine is superior to another." He added, "Physicians should thoroughly explain the characteristics and differences of each vaccine and then decide on the appropriate vaccination method together with the patient." "PCV21 vaccine is expected to be introduced...Expectation for adult prevention effectiveness" In this context, the 21-valent pneumococcal conjugate vaccine (PCV21) is expected to be approved this year. Regarding this, Dr. Heo explained, "Theoretically, the PCV21 vaccine can prevent the broadest range of serotypes in adults," and added, "At the Infectious Diseases Society conference, attendees showed a preference for PCV21 among the 15-valent, 20-valent, and 21-valent options." PCV21 is distinguished from existing vaccines by excluding serotypes included in the original PCV7 and incorporating the most non-vaccine type (NVT) serotypes whose adult incidence has increased due to serotype replacement phenomena following vaccine use. Dr. Heo stated, "Considering even the indirect effects of pediatric vaccination, the 21-valent vaccine could be the ideal vaccine." He added, "However, what strategy will be most effective for adults will need to be determined through real-world data from future field use." Dr. Heo also emphasized the establishment of an evidence-based vaccine policy to improve the pneumococcal prevention environment in South Korea. In South Korea, PPSV23 is currently provided free of charge to adults aged 65 and older. However, with the emergence of new vaccines, a multi-faceted review is necessary. Dr. Heo pointed out, "As new pneumococcal vaccines continue to be introduced, we need to closely analyze the efficacy of existing vaccines and domestic usage data. When introducing new vaccines, cost-effectiveness must also be reviewed." He added, "However, to respond to diverse serotype distributions and serotype changes resulting from vaccine use, a pneumococcal vaccine covering a broader range of serotypes is needed." Finally, Dr. Heo suggested, "While expanding the adult NIP is not easy at the moment due to cost issues, we have no choice but to follow the trend as vaccine technology advances. Systematic policy preparation considering complex factors is necessary."