- LOGIN
- MemberShip
- 2025-12-23 19:30:32
- Company
- Will Lixiana hit the 100 billion won mark for the NOAC RX?
- by Kim, Jin-Gu Nov 10, 2023 05:19am
- Attention is focused on whether Daiichi Sanko Lixiana will become the first NOAC drug to exceed 100 billion won in annual prescription sales. As of the third quarter, the cumulative prescription amount was 78.2 billion won, and if this trend continues, it is expected that prescriptions will easily exceed 100 billion won by the end of the year. Lixiana's annual prescription volume exceeded KRW 100 billion, making it the only original company to grow According to UBIST, a pharmaceutical market research firm, on the 9th, Lixiana's outpatient prescription volume in the third quarter was 26.8 billion won. Compared to 24.9 billion won in the third quarter of last year, it increased by 8% in one year. Lixiana's cumulative prescription amount in the third quarter was 78.2 billion won. The pharmaceutical industry predicts that if this trend continues, the number of prescriptions will increase to more than 100 billion won by the end of the year. Lixiana recorded prescriptions worth 96.7 billion won last year. In Korea, products that recorded over 100 billion won in prescriptions last year include Viatris 'Lipitor', Hanmi Pharmaceutical 'Rosuzet', HK inno. N 'K-CAB', Daewoong Bio Gliatamin, Handok 'Plavix', Chongkundang Gliatirin, LG Chemical Zemimet, etc. There are only 7. Even though the domestic NOAC market has reached a plateau, Lixiana is the only original drug that continues to see an upward trend in prescription performance. The NOAC market continued to grow rapidly until last year. The NOAC market, which was 147.2 billion won in 2018, reached 225.9 billion won in 2021, reaching the 200 billion won mark. Last year, the market size further expanded to 242.5 billion won. However, on a quarterly basis, it appears to have slowed down since peaking in the second quarter of last year. The NOAC market, which was 61.8 billion won in the second quarter of last year, decreased to 58.3 billion won in the 4th quarter. This year, it stagnated at 59.3 billion won in the first quarter, 60.2 billion won in the second quarter, and 60.9 billion won in the third quarter. BMS Eliquis, the second-largest product in the market, recorded prescription sales of 19.2 billion won in the third quarter. It remained at a similar level to the third quarter of last year. Eliquis has been recording prescription sales of around 19 billion won every quarter since the third quarter of last year. Xarelto's prescription performance decreased significantly due to the release of generics and the subsequent price reduction. Xarelto prescriptions in the third quarter amounted to 7.6 billion won, a 36% decrease from 11.8 billion won in the same period last year. Xarelto had issued quarterly prescriptions worth more than 15 billion won until the third quarter of 2021, but prescriptions have plummeted since the launch of generics. In the fourth quarter of last year, the quarterly prescription amount fell below 10 billion won and has been steadily decreasing since then. Boehringer Ingelheim Pradaxa's slump is prolonged. Pradaxa's prescription volume in the third quarter was 2.5 billion won, a 19% decrease compared to the same period last year. Generics for Xarelto are rapidly expanding their influence. The combined prescription amount for generics for Xarelto in the third quarter was 4.7 billion won. It increased by 72% compared to 2.7 billion won in the third quarter of last year. Its share in the rivaroxaban treatment market increased to 38%. Compared to 18% in the third quarter of last year, the market share has more than doubled in one year. Xarelto generics were released in large numbers in the third quarter of that year following the release of Chong Kun Dang's Riroxia in the second quarter of 2021. Chong Kun Dang's Riroxia, Hanmi Pharmaceutical's Riroxban, and Samjin Pharmaceutical's Rivoxaban are competing. As of the third quarter of this year, Riroxia's cumulative prescriptions were the highest at 3.3 billion won. Riroxban follows this at 2.4 billion won and Rivoxaban at 2.1 billion won. The remaining 30 companies had cumulative prescriptions of less than 1 billion won in the third quarter.
- Company
- Will Trodelbi become a second-line standard tx
- by Nov 09, 2023 05:43am
- Gilead Sciences Korea held a press conference at the Plaza Hotel in Seoul on the 7th to commemorate the domestic launch of Trodelbi, a treatment for metastatic triple-negative breast cancer Antibody Drug Conjugate (ADC) anticancer drugs first appeared in the domestic triple-negative breast cancer treatment market. To date, there have been no treatment options targeting triple-negative breast cancer treatment other than immunotherapy drugs and PARP inhibitors. Gilead is aiming to make Trodelbi the standard treatment option in this area. Gilead Sciences Korea held a press conference at the Plaza Hotel in Seoul on the 7th to commemorate the domestic launch of Trodelbi, a treatment for metastatic triple-negative breast cancer. Trodelbi is an ADC that targets Trop-2 protein, which is frequently observed on the surface of breast cancer cells and is used as a treatment for advanced or metastatic triple-negative breast cancer. Last May, Trodelbi was approved in Korea for the treatment of unresectable locally advanced or metastatic triple-negative breast cancer that has previously received two or more systemic treatments, at least one of which was for metastatic disease, and was launched in Korea last month. The phase 3 ASCENT study served as the basis for approval. The study was conducted to compare the effectiveness and safety of Trodelbi and chemotherapy in 529 patients with locally advanced or metastatic triple-negative breast cancer who had previously received chemotherapy twice or more. 12% of all patients had brain metastases. The primary endpoint was PFS in patients without brain metastases compared to baseline. Secondary endpoints included overall patient PFS, OS, and ORR. As for clinical results, the median PFS in patients without brain metastases, which was set as the primary endpoint, was recorded by Trodelbi at 5.6 months. This was a higher figure than the 1.7 months recorded for chemotherapy. The OS, measured regardless of the secondary endpoint of brain metastasis, was 11.8 months for Trodelbi and 6.9 months for chemotherapy in the entire patient group. There was a significant difference in ORR, with Trodelbi being 31% and chemotherapy being 4%. In terms of safety, serious adverse reactions such as neutropenia (7%), diarrhea (4%), and pneumonia (3%) occurred during Trodelbi administration. Treatment discontinuation due to adverse reactions was calculated to be 5% for Trodelbi and chemotherapy. Professor Son Joo-hyuk of the Department of Oncology at Yonsei Cancer Hospital said, “Trodelbi is considered a docile anticancer drug. The basic principle is that cytotoxic anticancer drugs kill cancer cells, and as they bind to antibodies, we confirmed a good safety profile without severe toxicity,” he said. “Trodelbi is an anticancer drug that should be used as a standard treatment for metastatic triple-negative breast cancer.” “I think it should be included not only in ESMO or NCCN but also in our country’s treatment guidelines,” he said. According to the NCCN guidelines, Trodelbi is classified as Category 1 for the second-line or higher treatment of adult patients with metastatic triple-negative breast cancer. Rare cancer, triple-negative breast cancer, need for secondary treatment options after treatment failure. Triple-negative breast cancer is classified as a rare cancer among breast cancers. Triple-negative breast cancer, which is negative for human epidermal growth factor receptor type 2 (HER2), hormone receptor (HR), and estrogen, is experiencing difficulties in developing targeted treatments. In particular, compared to HR- and HER2-positive breast cancer, there is a shortage of treatments and treatment outcomes are not as good. In the case of early triple-negative breast cancer, immunotherapy drugs can be used without biomarker analysis. However, in metastatic triple-negative breast cancer, it can only be used if there is PD-L1 expression. If BRCA mutation is confirmed, PARP inhibitors can be used, but if there is no corresponding biomarker mutation, there is no treatment. Professor Kim Ji-hyung of the Department of Oncology at Gangnam Severance Hospital said, “Triple-negative breast cancer has aggressive clinical manifestations and there are almost no targeted therapies available. “The disease-free survival rate is usually only 2 to 3 months, and there is no standard treatment,” he said. “Immunotherapy drugs also require confirmation of the PD-L1 expression rate, so treatment options are limited. “An effective treatment is needed for patients with metastatic triple-negative breast cancer who have failed primary treatment,” he said.
- Company
- Prescriptions of HCV market leader Mavyret halves in 1 yr
- by Kim, Jin-Gu Nov 09, 2023 05:43am
- Pic of Outpatient prescription performance of ‘Mavyret,’ the No. 1 product in the hepatitis C treatment market, has shrunk to less than half in just 1 year. In addition to the decrease in overall market size due to the decline in hepatitis C patients, Mavyret’s newly released competitors 'Epclusa' and ‘Vosevi’ have been rapidly expanding their influence in the market. According to the market research institution UBIST on the 8th, outpatient prescriptions of Mavyret in Q3 amounted to KRW 3.7 billion. This is a 52% decrease in 1 year compared to the KRW 7.8 billion it had posted in Q3 last year. Mavyret is a pan-genotypic hepatitis C treatment that was released by AbbVie. Since its release in Q3 2018, it quickly replaced Sovaldi and Harvoni which previously led the market. ‘Mavyret.’ can be used for patients with hepatitis C virus types 1 to 6, while Harvoni cannot be used for types 3 and Sovaldi cannot be used for types 5 and 6. The long treatment period of 12 weeks for Harvoni and Sovladi, compared to 8 weeks for Mavyret, also served as a weakness for Harvoni and Sovaldi. Mavyret’s share in the HCV treatment market share rose vertically from 3% in Q3 2018 to 56% in Q4. Subsequently, its market share exceeded 60% in Q1 2019 and then 70% in Q2 of the same year. Subsequently, its market share steadily increased and expanded to more than 80% in Q3 last year. Contrary to its expanded market share, prescription performance has been declining since 2019. Mavyret’s prescription performance, which recorded KRW 41.5 billion in 2019, decreased for 3 consecutive years to KRW 34.4 billion in 2020, KRW 32.8 billion in 2021, then to 31.2 billion won in 2022. Quarterly prescription performance of major hepatitis C treatments (Unit: KRW 100 million, Data UBIST) The analysis is that this is due to the steadily shrinking overall market size. The size of the HCV treatment market has steadily decreased from KRW 135.3 billion in 2017 to KRW 73.7 billion in 2018, then to KRW 65.1 billion in 2019, and KRW 47.4 billion in 2020, KRW 35.1 billion in 2021, then to KRW 34.2 billion in 2022. Compared to 2017, when the market had expanded to its maximum, the market had shrunk to a quarter in 5 years. The industry pointed to the characteristics held by the HCV treatments as a cause of the market contraction. Before the introduction of direct-acting antivirals (DAAs) like Mavyret, HCV had been a very critical disease. However, the treatment effect of HCV drugs had increased dramatically with the introduction of BMS’s Daklinza and Sunvepra. With Gilead Sciences' Sovaldi and Harvoni, MSD’s Zepatier, and Abbvie’s Mavyret that followed, the treatment effect had further increased. Ironically, as these new treatments offer an effect high enough to be close to a complete cure, the market size quickly contracted with the number of patients being prescribed the drug increasing within the finite number of patients in the market. As a result, the market size is shrinking. Pic of The entry of its competitors also affected the decline in Mavyret’s prescription performance. Gilead Sciences released Epclusa and Vosevi as next-generation hepatitis C treatments in November last year. The combined prescription performance of the two products is on the rise, reaching KRW 300 million in Q4 last year, then KRW 1.6 billion in Q1 of this year, KRW 2.7 billion in Q2, then to KRW 2.5 billion in Q3. During the same period, the market share of the two products had rapidly expanded from 4% to 19%, 34%, then 39%. If the current trend continues, it is predicted that it will surpass Mavyret and become a leader in the hepatitis C treatment market within the next year. However, like Mavyret, the fact that the overall market size is shrinking is expected to become a concern for Epclusa and Vosevi as well.
- Company
- Velexbru can be prescribed at general hospitals
- by Eo, Yun-Ho Nov 09, 2023 05:43am
- Velexbru, a new drug for lymphoma, can be prescribed at general hospitals. According to related industries, Ono Pharmaceutical's BTK (Brutons Tyrosine Kinase) inhibitor Velexbru recently passed the Drug Committee of top general hospitals such as AMC and Sinchon Severance Hospital. In addition, major medical institutions, including Samsung Seoul Hospital, are also conducting landing procedures. Velexbru was approved domestically in 2021 by the Ministry of Food and Drug Safety as a monotherapy for patients with Primary Central Nervous System Lymphoma. This drug is the first BTK inhibitor approved in Korea for the treatment of patients with relapsed or refractory B-cell primary central nervous system lymphoma for which there is no standard treatment. The effectiveness of Velexbru was confirmed through ONO-4059-02, a non-blinded, uncontrolled phase 1/2 clinical study of Velexbru conducted in Japan in patients with relapsed or refractory primary central nervous system lymphoma. A total of 44 patients were enrolled in the study and were administered Velexbru 320 mg (80 mg per tablet, n = 20), 480 mg (n = 7), and 480 mg (n = 17) on an empty stomach orally once daily. Drug administration continued until the disease progressed or unacceptable toxicity occurred. The primary endpoint was ORR according to BICR. As a result of the study, the ORR for the approved dosage and dosage of 480 mg (fasting) was 52.9%. Major adverse reactions corresponding to grades 3 and 4 included neutropenia, leukopenia, and hypertriglyceridemia, which occurred in 11.8% of patients each. PCNSL is a very rare disease, so it is quite difficult to estimate the nationwide patient population. In general, it is reported that approximately 6,000 lymphoma patients occur annually in Korea, and about 2,000 primary brain tumors occur annually. Among these, PCNSL is known to account for approximately 2% of all brain tumor patients. Considering that PCNSL is reported to occur at 0.44 cases per 100,000 people in Western countries, it is estimated that approximately 200 to 250 new PCNSL patients will occur annually in Korea.
- Company
- Whether Ilaris for 10 will receive reimb gains attention
- by Eo, Yun-Ho Nov 08, 2023 05:37am
- Attention is focused on whether progress will be made on discussions for the insurance reimbursement of 'Ilaris', a treatment that is used by around ten patients in Korea. According to industry sources, reimbursement discussions for Novartis Korea’s Ilaris (canakinumab) are currently being negotiated between the government and pharmaceutical companies at the Drug Reimbursement Standards Subcommittee stage. Half a year had passed since the company reapplied for reimbursement in line with the addition of its pediatric indications in April at registration. The company had failed to list the drug for reimbursement two times. A bumpy road lies ahead for its reimbursement still. The current focus of Ilaris’s reimbursement discussion is whether or not the drug can be applied the pharmacoeconomic evaluation exemption system. Ilaris's listing will become more difficult if its reimbursement evaluation is not carried out through the PE exemption track, Ilaris was initially approved in 2015 as a hereditary recurrent fever syndrome treatment. Its disease type is specified according to the mutated gene. Among the various specific syndromes that accompany hereditary recurrent fever, Ilaris is approved in Korea to treat ▲Cryopyrin-Associated Periodic Syndromes (CAPS), ▲Tumor Necrosis Factor Receptor Associated Periodic Syndrome (TRAPS), ▲Hyperimmunoglobulin D Syndrome (HIDS)/Mevalonate Kinase Deficiency (MKD), ▲ Familial Mediterranean Fever (FMF), and ▲ Systemic juvenile idiopathic arthritis (JIA). Among these, CAPS is further classified into ▲Familial Cold Autoinflammatory Syndrome FCAS)/ Familial Cold Urticaria (FCU), ▲Muckle-Wells Syndrome (MWS), ▲Neonatal Onset Multisystem Inflammatory Disease (NOMID)/Chronic Infantile Neurologic Cutaneous and Articular. Due to the very small number of subject patients and complex indications, it was difficult to progress discussions for its reimbursement. The number of patients who are applied the various indications for Ilaris is extremely small. Some indications for Ilaris do not even have disease codes or are only recently registered. In fact, the number of domestic patients that can use Ilaris is estimated to be around 13. Due to Ilaris’s non-reimbursement, patients have been receiving a treatment they can with an alternative that is not really an alternative. Patients with CAPS have been using ‘Kineret’ which is being supplied through the Korea Orphan & Essential Drug Center. As this drug has not been officially approved by the Ministry of Food and Drug Safety and is being supplied through the center, when supply disruptions occur, no entity in Korea has the obligation to ensure its supply, rendering the communication channel unclear and limits to improving its supply. In the case of FMF, colchicine is recommended as a first-line treatment but is not available in Korea. Ilaris is approved for use in patients with FMF who are contraindicated, intolerant, or achieved inadequate response with the highest-dose colchicine. However, since colchicine has not been introduced domestically, it will be difficult for FMF patients to use Ilaris, even if it receives reimbursement if colchicine's approval and reimbursement issues are not resolved. To solve this problem, an academic society applied for non-reimbursed off-label use of colchicine and reimbursement extension for the drug. In addition, voices calling for Ilaris’s reimbursement listing had risen in the recent NA audit. The National Assembly’s Health and Welfare Committee audit of the National Health Insurance Service and the Health Insurance Review and Assessment Service, Rep Sun-woo Kang of the Democratic Party of Korea, said, "Patients are spending KRW 80 million to as much as KRW 100 million a year to purchase non-reimbursed drugs. We ask the government to promptly list the drug for reimbursement to improve the quality of life for children and ease the financial burden borne by the patients' families.” Therefore, it remains to be seen whether the company and health authorities can reach a consensus and achieve reimbursement listing for ‘Ilaris’, a drug for a very small number of patients.
- Company
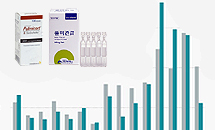
- Budesonide’s price to be raised due to increased demand
- by Chon, Seung-Hyun Nov 07, 2023 05:34am
- Health authorities are working to increase the price of asthma medications containing ‘budesonide’, which have been experiencing supply shortages. Quarterly prescriptions of budesonide, which was less than KRW 1 billion, more than tripled after the COVID-19 pandemic, intensifying the supply and demand imbalance. According to industry sources on the 6th, the Korean health authorities have been reportedly working to increase the prices of two drugs that contain the asthma treatment ingredient budesonide. The authorities are known to be reviewing a price increase due to the recent surge in their demand. Budesonide is used to treat bronchial asthma and acute laryngotracheobronchitis in infants and children. Two products, AstraZeneca's Pulmicort and Kuhnil Pharmaceutical's Pulmican, are currently available in the Korean market. According to the pharmaceutical research institution UBIST, the prescription amount for Pulmicort and Pulmican in Q3 this year was KRW 1.6 billion. The amount decreased by 0.2% from Q3 last year but is 155.8% in 2 years from the KRW 600 million in Q3 2021. In 2020, the prescription market for budesonide was only KRW 3.8 billion. The average quarterly prescription amount was less than KRW 1 billion. In 2021, prescription performance was less than KRW 1 billion from Q1 to Q3. Quarterly outpatient prescriptions of Pulmicort(grey) and Pulmican(green) Budesonide prescriptions increased 39.0% YoY to KRW 1.4 billion in Q4 2021, then soared again to KRW 3 billion in Q4 last year, recording an over threefold increase from 2 years ago. This year, budesonide continued to increase in sales, recording KRW 2.5 billion and KRW 2.8 billion in Q1 and Q2, respectively. The cumulative prescription amount for budesonide in Q3 this year was KRW 6.9 billion, approximately 3 times higher than KRW 2.3 billion during the same period in 2021. The prescription market for budesonide has also increased significantly with the surge in the number of confirmed COVID-19 cases since the end of 2021. Recently, the demand for asthma drugs has increased due to the increase in not only confirmed cases of COVID-19 but also cold and flu patients, resulting in a supply-demand imbalance where supply cannot meet demand Prescriptions for Pulmican and Pulmicort both surged from the end of 2021. Pulmicort’s quarterly prescriptions from 2020 to Q3 2021 ranged around KRW 300 million to 500 million. It jumped 36.6% YoY to KRW 800 million in Q4 2021 and exceeded KRW 1 billion in the of last year. The cumulative prescription amount for Pulmicort in Q3 this year was KRW 3.4 billion, up 194.7% from the cumulative KRW 1.2 billion in Q3 2021. Pulmican’s quarterly prescriptions from Q1 2020 to Q3 last year fell below KRW 1 billion. However, sales surged to KRW 1.8 billion in Q4 last year, and recorded KRW 1.5 billion and KRW 1.4 billion in Q1 and Q2 this year, respectively. The cumulative prescription amount of Pulmican in Q3 this year was KRW 3.4 billion, a threefold increase from 2 years ago. If raised, budesonide will become the fourth drug to receive a price increase since last year, following acetaminophen, magnesium hydroxide, and pseudoephedrine. The Ministry of Health and Welfare raised the insurance price ceiling of 18 acetaminophen 650mg items by up to 76.5% in December last year. The insurance price limit for 650mg acetaminophen, which ranged between KRW 43 to 51 before then, was raised to KRW 90. The government made an unprecedented decision to raise the price of all acetaminophen together when pharmaceutical companies expressed reluctance to increase production due to the drug’s poor cost structure. However, it is a temporary increase that will be adjusted to KRW 70 from December this year. Pharmaceutical companies had promised to increase production of acetaminophen in line with the price hike. The Ministry of Health and Welfare has also raised the price of magnesium hydroxide-based laxatives since last June. The price of Magmil was raised by 27.8% from KRW 18 to KRW 23. Cho-A Pharmaceutical's Marogel was raised from KRW 15 to KRW 22, and Sinil Pharm’s M Tab Sinil was raised from KRW 16 to KRW 22. Last month, the prices of the 4 types of pseudoephedrine single-agent drugs were increased by up to 45%. The insurance price of Sinil Pharm’s Pseudoephedrine Tab Sinil increased by 45% from KRW 20 to KRW 29. The price of Sam Il Pharmaceutical's Sudafed rose 39% from KRW 23 to KRW 32. The insurance drug price of Sama Pharm’s Schdafen and Kolon Pharmaceutical's Cosue was raised by more than 30% from KRW 23 to KRW 30 and KRW 31, respectively.
- Company
- Chong Kun Dang signs KRW 1.7 tril deal with Novartis
- by Kim, Jin-Gu Nov 07, 2023 05:34am
- On the 6th, Chong Kun Dang Pharm announced on the 6th that it signed a licensing out agreement worth KRW 1.7 trillion for its new drug candidate 'CKD-510' with the global pharmaceutical company Novartis. Under the agreement, Novartis will have exclusive rights to the development and commercialization of CKD Pharm’s CKD-510 worldwide, excluding Korea. CKD Pharm will first receive an upfront payment of USD 80 million (approximately KRW 106.1 billion), followed by milestone payments of USD 1,225 billion (approximately KRW 1.624 trillion) for future development and approval milestones, in addition to sales royalties based on net sales CKD-510 is a new drug candidate in the low molecular substance histone deacetylase 6 (HDAC6) inhibitor class being developed by CKD Pharm. It has applied a highly selective non-hydroxamic acid (NHA) platform technology. It had confirmed its efficacy in a preclinical study in several HDAC6-related diseases including cardiovascular disease. Also, it demonstrated safety and tolerability in Phase 1 trials conducted in Europe and the United States. Young-Joo Kim, CEO of CKD Pharm, said, “We have previously licensed out the anemia drug biosimilar ‘Nesbell,’ and new antidiabetic drug ‘Duvie’ in Japan and the US, respectively. This is the largest agreement ever made by the company and is the result of our continuous investment with over 12% R&D to sales.” Mi-Yeop Lee, Head of CKD Pharm’s Product Development Division, said, “We expect Novartis will develop CKD-510 into a global new drug based on Novarits’s long new drug development know-how and commercialization capabilities. Using the agreement as momentum, CKD Pharm will spur clinical development of its new drug candidates to achieve a result as soon as possible.” CKD Pharm plans to use its own HDAC6 platform to continue developing treatments for various diseases in the future. In addition, the company is speeding up the development of a new bispecific antibody anti-cancer bio drug 'CKD-702' and a dyslipidemia treatment 'CKD-508', which are currently in Phase 1 trials. In addition, the company plans to expand the scope of new drug development to advanced biopharmaceuticals such as gene therapy and ADC anticancer drugs to develop first-in-class new drugs and drugs with unmet needs.
- Company
- First GIFT drug Lunsumio in reimb process after approval
- by Eo, Yun-Ho Nov 07, 2023 05:33am
- The new lymphoma drug ‘Lunsumio' started the insurance reimbursement process immediately after its approval in Korea. According to industry sources, the anti-CD20/CD3 T-cell engaging bispecific antibody Lunsumio (mosunetuzumab) is starting the listing process using the approval-reimbursement evaluation linkage system. Lunsumio is the first product that was designated for the Global Innovative Product on Fast Track (GIFT) program. It received approval by the Ministry of Food and Drug Safety on the 3rd. The GIFT system supports the rapid commercialization of drugs for which there are no other existing treatment options, such as those aimed at treating life-threatening serious diseases or rare diseases. Lunsumio was considered a 'drug without an existing treatment’ and was designated as the first product for the GIFT program in November 2022. In addition to the GIFT designation, Roche sought more efficient market access by applying for an approval-reimbursement linkage system. Accordingly, attention is being paid to whether Lunsumio, the first GIFT drug, can produce significant results. Lunsumio can be prescribed to treat adult patients with relapsed or refractory follicular lymphoma after two or more lines of systemic therapy. Follicular lymphoma is a type of non-Hodgkin Lymphoma (NHL) that occurs when cells in the lymphoid tissue become malignant. Because the symptoms are mild and progress slowly, approximately 80% of cases are discovered in Stage 3 or 4 after disease progression and show worse progression with relapse. Although the median progression-free survival (mPFS) for first-line treatment patients is 10.6 years but is reduced to 2 years in third-line treatment patients. Lunsumio is a first-in-class anti-CD20/CD3 T-cell engaging bispecific antibody for relapsed or refractory follicular lymphoma and is designed to target CD20 on the surface of B cells and CD3 on the surface of T cells. The dual targeting activates and redirects a patient’s existing T cells to engage and eliminate target B cells by releasing cytotoxic proteins into the B cells. It was released as a finished product and can be administered immediately without waiting for the treatment manufacturing process, and outpatient treatment is possible without the need for hospitalization. The administration period is fixed to 8 cycles, and if the patient does not achieve complete remission during this period, it can be administered up to 17 cycles. Seok Won Kim, Professor of Hematology-Oncology, said, “Follicular lymphoma is considered a benign lymphoma with a life expectancy of up to 20 years, but the disease becomes more aggressive and the prognosis worsens with repeated relapses. Therefore, there is an urgent need for an effective treatment option that can provide a cure for patients who have relapsed more than twice.
- Company
- Scemblix may be prescribed at Big 5 Hospitals in KOR
- by Eo, Yun-Ho Nov 06, 2023 05:27am
- The next-generation chronic myeloid leukemia drug ‘Scemblix’ can now be prescribed at general hospitals in Korea. According to the industry sources, Novartis Korea's Philadelphia chromosome-positive chronic myeloid leukemia (Ph+CML) treatment ‘Scemblix (asciminib)' has passed the drug committees of the Big 5 tertiary hospitals in Korea- Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary’s Hospital, Asan Medical Center, Sinchon Severance Hospital – as well as major medical institutions nationwide. As the drug was listed for reimbursement in June, the prescription environment seems to have been created quite quickly. Scemblix is reimbursed as a treatment for patients aged 18 and older with Ph+ CML in the chronic phase who are resistant or intolerant to two or more prior tyrosine kinase inhibitors (TKIs). However, Scemblix is only allowed reimbursed for patients without the T315I or V299L mutations in Korea. Meanwhile, Scemblix was approved in June last year as a treatment for adult patients with Ph+ CML in the chronic phase previously treated with two or more tyrosine kinase inhibitors (TKIs). Chronic myeloid leukemia is a malignant blood disorder that occurs when myeloid cells produce white blood cells. Although it progresses slowly, if left untreated, it can gradually progress to acute leukemia, and result in spleen enlargement and frequent infections as well as bleeding. Currently, TKIs are used to treat patients with chronic myeloid leukemia, but treatment may be limited due to intolerance or resistance, and the longer the treatment period, the higher the failure rate. Research results show that up to 70% of patients treated in the second line did not achieve major molecular response (MMR) within 2 years. Contrary to existing TKIs that had the potential to develop resistance due to mutations in the ATP binding site, Scemblix specifically binds to the ABL1 myristoyl pocket to overcome mutations at the defective BCR::ABL1 gene, which is associated with the over-production of leukemic cells., and is also called the STAMP (Specifically Targeting the ABL Myristoyl Pocket) inhibitor. Through the mechanism of action, it shows high specificity for BRC-ABL1 and is unlikely to cause resistance due to mutations in the BCR-ABL1 gene, which is associated with resistance and intolerance in patients with chronic myeloid leukemia that used to occur with existing treatments. Meanwhile, Scemblix demonstrated its efficacy through the Phase III ASCEMBL study, which confirmed its clinical utility and safety profile in patients with chronic phase Philadelphia chromosome-positive chronic myeloid leukemia who received at least two or more TKI treatments. Study results showed that Scemblix improved the rate of major molecular response (MMR) compared to its comparator bosutinib by 2 times. Also, the rate of treatment discontinuation due to adverse reactions in the Scemblix group was 5.8%, about one-fourth of the control group's 21.1%, confirming its overall safety profile.
- Company
- Wegovy and Mounjaro begin to dominate the obesity market
- by Kim, Jin-Gu Nov 06, 2023 05:27am
- Wegovy and Mounjaro Novo Nordisk Wegovy and Eli Lilly Mounjaro have grown explosively in the global market. The two products together generated sales of close to 4 trillion won by the third quarter. In the future, if Mounjaro officially obtains an obesity treatment indication from the FDA, competition between the two products is expected to become more intense. The obesity market rises vertically to Wegovy 4.1 trillion won and Mounjaro 3.9 trillion won According to the pharmaceutical industry on the 4th, Novo Nordisk's obesity treatment Wegovy recorded cumulative sales of approximately 4.1248 trillion won in the third quarter of this year. Sales increased nearly six times compared to the same period last year. Wegovy is a Semaglutide GLP-1 receptor agonist drug. Novo Nordisk first developed Ozempic as a diabetes treatment with the same ingredient and then relaunched it as Wigobi after proving its effectiveness in treating obesity. Since its launch, Wegovy has received a lot of attention. In the U.S. market, demand was high enough to cause shortages within a few months of launch. Thanks to Wegovy's sensation, Novo Nordisk's sales also increased significantly. Novo Nordisk's total cumulative sales in the third quarter amounted to approximately 31.67 trillion won, an increase of 33% in one year. Eli Lilly Mounjaro, which is considered a potential competitor to Wegovy, also posted sales close to 4 trillion won. According to the third-quarter performance data released by Eli Lilly on the 2nd (US local time), Mounjaro's cumulative sales in the third quarter amounted to $2.957.5 billion (approximately 3.91 trillion won). Compared to the same period last year, sales rose vertically by more than 14 times. It is estimated that most of the sales come from off-label prescriptions aimed at treating obesity. Currently, Mounjaro has been approved by the U.S. FDA as a treatment for diabetes. However, as the effectiveness of this product in treating obesity became known, it received great attention and was widely prescribed off-label in the United States. It contributed significantly to Lilly's overall sales growth. Lilly's cumulative sales in the third quarter were $24.77 billion (about 32.77 trillion won), a 16% increase compared to $21.2936 billion in the third quarter of last year. It is analyzed that although the sales of Trulicity, Lilly's highest-selling product, decreased by 7% due to supply instability, this was offset by the significant increase in sales of Mounjaro. Mounjaro will compete with Wegovy in earnest once its official obesity treatment is approved. Attention is focused on the timing of Mounjaro's official approval as an obesity treatment. Lilly has proven its weight loss effect through a separate phase 3 obesity clinical trial and is currently going through the approval process for obesity indications. Lilly expects to receive additional approval for obesity indications from the U.S. FDA within this year. If Mounjaro is officially approved as an obesity treatment within the year as Lilly plans, full-scale competition with WeGobee is expected after next year. Although there are no studies directly comparing the two drugs, looking at each clinical trial alone, Maunzaro's weight loss effect is evaluated to be slightly superior. In Mounjaro's SURMOUNT-1 study, the weight loss effect was found to be 15.0-20.9% depending on the drug dose. This study was conducted on 2,539 adults with a body mass index (BMI) of 30 or higher or a BMI of 27 or higher who suffered from one or more weight-related complications other than diabetes. When they were administered 5mg, 10mg, and 15mg of Mounjaro for 72 weeks, the weight loss rates were 15.0%, 19.5%, and 20.9%, respectively. Wegovy's STEP study showed a weight loss effect of 14.9%. The study was conducted on 1,961 adults with a BMI of 30 or higher or a BMI of 27 or higher who suffered from one or more weight-related diseases other than diabetes. When these people were administered Wegovy 2.4mg for 68 weeks, the weight loss rate was 14.9%. Additionally, the proportion of people who lost more than 5% of their body weight was 86.4%.