- LOGIN
- MemberShip
- 2025-12-22 20:44:54
- Company
- Anti-cancer drug trends changes from IV→SC
- by Moon, sung-ho Aug 30, 2024 05:50am
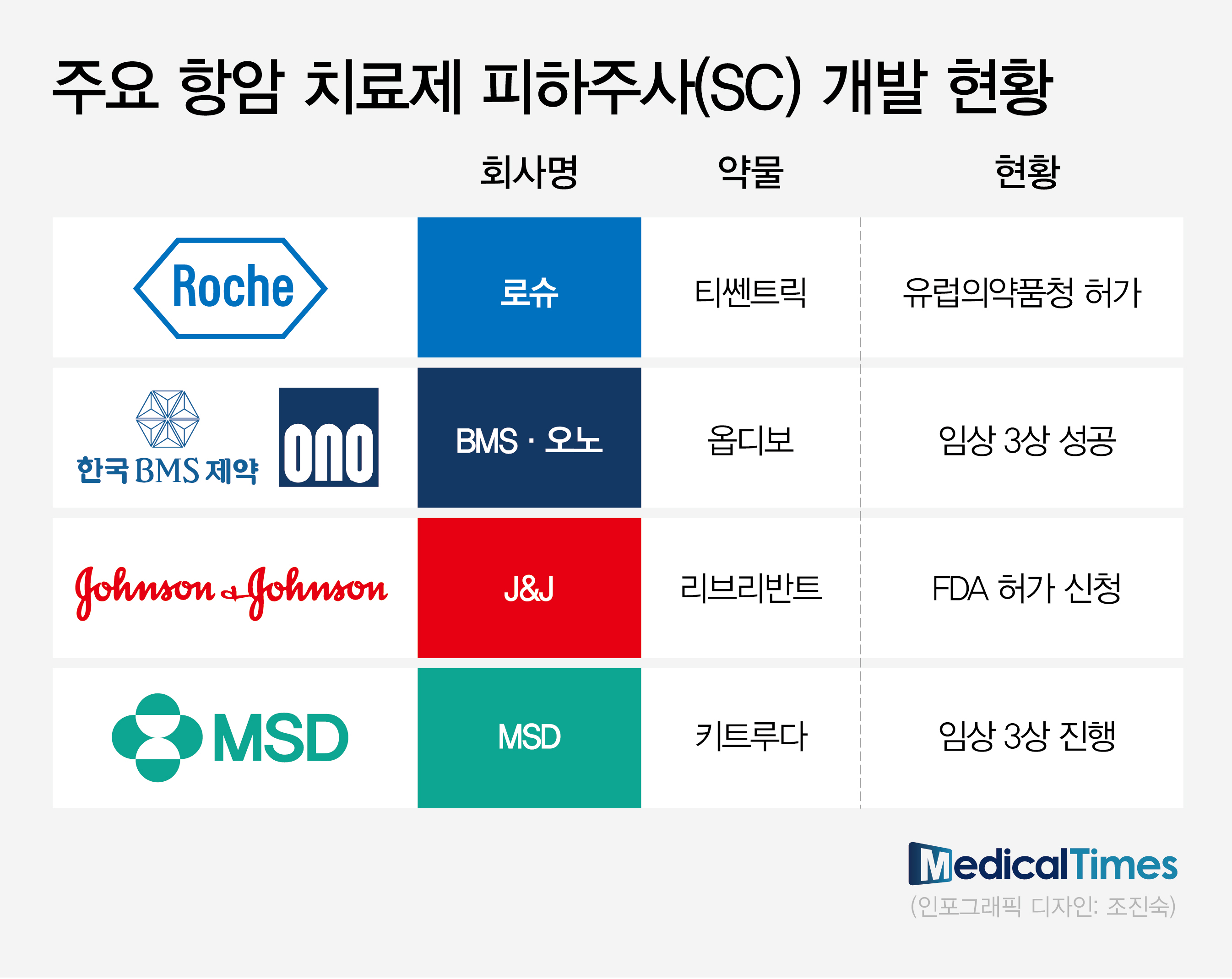
- Global pharmaceutical companies speed up the development of subcutaneous (SC) formulations of their proprietary intravenous (IV) medications. To overcome the disadvantage of IV formulation medications that have long administration duration, more anti-cancer drugs are shifting to SC formulations. Following the trend, Korean companies with SC formulation technology are gaining attention. Then, what can we predict about the success in real-world clinical practices? While the shift to SC formulation of anti-cancer drugs is trending, pharmaceutical‧biotech industries focus on real-world clinical practices. Global pharmaceutical companies with IV formulations of anti-cancer drugs are proactively conducting clinical trials to transition to SC formulation products. (Table) RocheAccording to pharmaceutical and biotech companies on August 24th, global pharmaceutical companies with proprietary IV formulations of anti-cancer drugs are proactively conducting clinical trials to transition to SC formulation products. SC formulation products are injected into the subcutaneous layer of the skin. Injection sites are typically arms, thighs, and abdominal region. Until now, anti-cancer drugs were available primarily as IV formulations, which inject medications into veins. IV formulation has the advantages of fast absorption and accurate administration but has the disadvantage of taking a long time. For IV injection of anti-cancer drugs, patients were burdened by having to visit hospitals and withstand four to five hours of needle insertion. In contrast, developing anti-cancer drugs as SC formulations has the advantage of substantially improving patient convenience of administration. The administration duration was reduced from a couple of hours to a maximum of ten minutes. Therefore, patients do not need to stay in the hospital for a long time for anti-cancer drugs. As a result, global pharmaceutical companies with immune checkpoint inhibitors are proactively conducting clinical trials and applying for approvals to transition to SC formulations. Following Roche's 'Tecentriq (atezolizumab)' SC obtaining marketing authorization from the European Medicines Agency (EMA) in January, BMS and Ono Pharmaceutical's R&D of 'Opdivo (nivolumab)' and 'Rybrevant (amivantamab)' is nearing the end. These immune checkpoint inhibitors share the same goal to defend against their sales decrease due to patent expiration. Furthermore, the development of SC formulation products has gained more attention since SC formulations have a greater advantage in patient access in the global market, especially in the U.S. market. Recently, J&J confirmed the non-inferiority of SC formulations compared to IV formulations through the Phase 3 PALOMA-3 study, presented at the American Society of Clinical Oncology (ASCO) meeting. Based on the results, J&J has recently applied for additional U.S. FDA approval on Rybrevant SC formulation. Professor Byoung Chul Cho (Director of the Lung Cancer Center at Yonsei Cancer Hospital) said, "The United States provides incentives to using injections, and the amount of incentives is the same between IV injectable or SC injectable," and explained, "There is no need to maintain IV formulation injectables, which commonly induce injection-associated adverse reactions." Professor Sun Min Lim (Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Hospital) also said, "SC injectables only take 1-2 minutes for Rybrevant administration. The common adverse reactions of IV injectables are fever and lowered blood pressure," and added, "In my opinion, SC injectables can reduce such adverse reactions." In the global market trend, clinical practices in South Korea are accelerating the introduction of SC formulation products, which overcome the previous disadvantages of IV formulation. For example, Roche's Phesgo has been recently introduced with reimbursement. Phesgo is an anti-cancer drug developed by changing the IV formulations of Herceptin (trastuzumab) and Perjeta (pertuzumab) to SC formulations. It received approval in September 2021 as the first investigational biopharmaceutical. Phesgo was developed by combining two IV formulation products into SC formulation. It is known to decrease the administration duration for patients with breast cancer substantially. It has been designated as an innovative new drug (IND) and is reimbursable, leading to its recent use in clinical practices. If patients with metastatic HER2-positive breast cancer who have been receiving the maintenance therapy, IV formulations of Herceptin plus Perjeta every three weeks, were to change to Phesgo SC, administration and monitoring duration is expected to decrease from over four hours to 20 minutes. The remaining step is whether the SC formulation can be used in clinical practices in South Korea. While there are clear advantages in patient administration, there are opinions that, unlike in the global market, such as the U.S., it is challenging to quickly replace the existing market in South Korea due to geographic accessibility. Additionally, many believe it will be challenging for healthcare professionals to readily switch to the SC formulation, which they have yet to experience. However, some argue that the fact that most cancer drug administrations are carried out primarily in large hospitals and are performed in the same way in injection rooms is more positive. The large number of patients who can receive treatment may facilitate the rapid establishment of the SC formulation. Professor Park Yeon Hee (Division of Hematolology-Oncology at Samsung Medical Center) said, "Korean patients tend to wait in the hospital, and there are long waits in large hospitals. Therefore, patients may prefer switching to SC formulations," and stated, "In clinical practices, patients may prefer IV injection despite the wait as SC prescriptions, other than clinical studies, have only been made recently."
- Company
- K-bio jumps into developing new drugs for pancreatic cancer
- by Son, Hyung-Min Aug 30, 2024 05:50am
- Korean pharmaceutical and biotech companies have jumped into the development of new drugs for pancreatic cancer, which is categorized as refractory cancer. Prestige Biopharma, New Cancer Cure Bio (NCC-Bio), LigaChem Biosciences, and Aptamer Sciences are conducting clinical trials to challenge the field. These companies plan to investigate the commercialization potential of their candidates through antibody-drug conjugates (ADCs), targeted anti-cancer agents, and new antibody drugs. New ADC drugs target TROP2 for treating pancreatic cancer According to industry sources on August 29th, Aptamer Sciences applied for a patent for its 'Aptamer-Drug Conjugate (ApDC)' and commenced treatment development. ApDC is a next-generation ADC new drug development platform with its proprietary branched linker-payload technology. Pancreatic cancer is known to have the lowest survival rate among cancer disorders. The five-year survival rate of pancreatic cancer is merely 15.9% from 2017 to 2021, according to the National Cancer Center. The early detection rate of pancreatic cancer is less than 10% due to the location of the organ, and the cancer readily metastasizes to peripheral organs. Until now, several domestic and global companies have jumped into developing new drugs for pancreatic cancer but mainly failed in clinical trials. Aptamer Sciences and latecomer companies plan to develop new drugs for pancreatic cancer using their new drug candidates, such as ADC. Aptamer Sciences derived 'AST-203' using the ApDC platform. AST-203 is made by conjugating TROP2-targeting antibody with 'MMAE,' a microtubule disruption agent, with linker 'VC-PAB.' TROP2 is an intracellular calcium signal transducer that regulates cell proliferation and survival. Although this protein is found in healthy cells, it is often overexpressed in cancer cells and is associated with drug resistance. The only TROP2-targeting new drug available in the market is Gilead Sciences' ADC Trodelvy, which is approved for treating triple-negative breast cancer. TROP2 is commonly found in breast cancer, non-small cell lung cancer (NSCLC), colorectal cancer, and pancreatic cancer. The clinical trials for latecomer agents are being conducted to target major solid cancers. In pre-clinical trials, Aptamer Sciences has confirmed the potential of AST-203 in a tumor spheroid model (3D cell culture of spheroids). According to the company, AST-203 showed a 6.7-fold higher tumor penetration rate than an existing therapy, Trodelvy. Additionally, in a pancreatic cancer animal model, AST-203 demonstrated dose-dependent tumor growth suppressive effects and tumor regression, reducing tumor sizes in all experimental animal groups. Aptamer Sciences aims to commence a clinical trial for AST-203 in two years. LigaChem Biosciences (hereafter referred to as LigaChem Bio) is developing TROP2-targeting ADC LCB84. Last year, LigaChem Bio successfully signed a deal with Janssen, a subsidiary of U.S.-based Johnson & Johnson (J&J) to license-out its LCB84. LCB85 consists of LigaChem Bio's proprietary ConjuAll linker and four MMAE, a microtubule disruption agent. ADC consists of a linker, payload (drug conjugate), and antibody. The ConjuAll linker is known to overcome the issue of releasing cytotoxic drugs into the blood and attacking healthy cells. In preclinical studies, LCB84 demonstrated effects in solid tumors not responding to topoisomerase enzymes-based TROP2 ADC payloads. Major ADCs like Enhertu (trastuzumab deruxtecan) incorporate the technology of topoisomerase enzymes. LigaChem Bio received Investigational New Drug (IND) approval from the U.S. Food and Drug Administration (FDA) in June, and the company is currently conducting Phase1/2 studies in the United States. In clinical trials, LigaChem Bio plans to investigate the preventive efficacy of LCB84 monotherapy and the combination therapy of LCB84 plus immune checkpoint inhibitor. Prestige Biopharma·NCC-Bio successfully enter clinical trials In addition to ADCs, various new drug candidates are being investigated for potential treatment of pancreatic cancer. Prestige Biopharma is developing a new antibody drug candidate, PBP1510. PBP1510 works by neutralizing pancreatic adenocarcinoma upregulated factor (PAUF), a protein target for the treatment of pancreatic cancer. The PBP1510 Phase 1/2a trials are being conducted in Spain, the United States, Singapore, and Australia. Through the trials, Prestige Biopharma plans to investigate the safety and tolerability of PBP1510 plus gemcitabine combination therapy. Prestige Biopharma aims to expand PBP1510's indication to ovarian cancer and prostate cancer in addition to pancreatic cancer. PAUF is known to be associated with ovarian cancer and prostate cancer. NCC-Bio will commence the development of a new drug for treating pancreatic cancer using 'KN510713,' a targeted anti-cancer agent and fatty acid oxidation (FAO) inhibitor. The company is conducting clinical trials after receiving approval for the KN510713 Phase 1 trial last year. NCC-Bio, founded by Kim Soo Youl, who used to work at the National Cancer Center, is a biotech company specializing in developing new anti-cancer drugs. KN510713 is being developed as an anti-cancer drug candidate that inhibits catabolism. Its clinical trial is the first anti-cancer agent trial to target inhibiting fatty acid oxidation (FAO) metabolism in cancer. KN510713 works by decreasing cancer cell growth by blocking the energy supply to tumor cells without affecting healthy ones. NCC-Bio is studying the last cohort of the Phase 1 trial and plans to enter the Phase 2 trial next year.
- Company
- Korean pharma and biotechs expand R&D investments
- by Chon, Seung-Hyun Aug 29, 2024 04:32am
- Listed pharma and biotech companies have expanded their research and development (R&D) investments in Korea this year. 3 out of 5 major pharmaceutical companies increased their R&D investments from last year to discover new candidates. Pharmaceutical companies with larger sales have been more active in reinvesting in R&D. According to the Financial Supervisory Service on Monday, the R&D investment expenses of 20 major pharma and biotech companies totaled at KRW 1.236 trillion in the first half of the year, up 12.7% from KRW 1.967 trillion a year earlier. The data was compiled from the top 20 pharmaceutical companies by revenue that submitted semi-annual reports. Ildong Pharmaceutical, which spun off its R&D subsidiary last year, was not included in the survey. Thirteen of the 20 major pharmaceutical companies increased their R&D investment in the first half of the year compared to the same period last year. Celltrion, Samsung Biologics, Daewoong Pharmaceutical, Yuhan Corp, Hanmi Pharmaceuticals, Dong-A ST, SK Biopharm, HK Inno. N, Boryung Pharmaceutical, Dongkook Pharmaceutical, Huons, Dongwha Pharmaceutical, and Celltrion Pharm increased their R&D spending in the first half of the year compared to the same period last year. Celltrion invested the most, KRW 206.7 billion in R&D in the first half of the year. This is a 37.3% expansion compared to that in the first half of last year. Celltrion has completed the development of biosimilars of Remicade, Enbrel, Mabthera, and Humira and is selling them in the U.S. and Europe. Celltrion has received approval for two biosimilars in Europe this year. In May, the company received marketing authorization from the European Commission for its first biosimilar of Xolair, Omlyclo. Xolair is an antibody biopharmaceutical agent used to treat allergic asthma, chronic rhinosinusitis with nasal polyps, and chronic idiopathic urticaria. It generated global sales of about KRW 5 trillion last year. Recently, Stekima, its biosimilar version of the autoimmune disease treatment Stelara, received European marketing authorization. Stelara is an autoimmune disease treatment for plaque psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis developed by Janssen. Celltrion has received 8 and 6 approvals for its biosimilars in Europe and the U.S., respectively. Samsung Biologics' R&D expenditure in the first half of the year increased 20.2% year-on-year to KRW 177 billion. Samsung Biologics' main business is contract manufacturing (CMO) and contract development (CDO) of raw materials for biopharmaceuticals. Samsung Biologics' R&D organization provides technical support for the production of customer products and cell line process R&D at the Manufacturing Science And Technology (MSAT) laboratory, CDO Development Center, and Bio R&D Center. The company’s R&D investments have also increased due to the increase in orders for biopharmaceutical contract manufacturing (CMO) and contract development (CDO) orders. Samsung Biologics' R&D investment also includes Samsung Bioepis’ R&D expenses. Since 2022, Samsung Bioepis has become a wholly-owned subsidiary of Samsung Biologics. Samsung Bioepis has received a total of 4 biosimilar approvals in the U.S. and Europe this year In April, Samsung Bioepis received marketing authorization for its Stelara biosimilar Pyzchiva in Europe. In May, the company received approval for Opuviz, a biosimilar to Eylea used for macular degeneration. Following the FDA approval of Pyzchiva in June, Samsung Bioepis received marketing authorization for the rare disease treatment Epysqli in July. Epysqli is a biosimilar product of Soliris that was developed by Alexion in the US. Among traditional pharmaceutical companies, Daewoong Pharmaceutical made the largest R&D investment of KRW 111.8 billion in the first half of the year. This is an 18.3% rise year-on-year. Daewoong is developing new drugs in areas such as ulcerative colitis, idiopathic pulmonary fibrosis, obesity, autoimmune diseases, and infectious diseases. It is also conducting joint research with HanAll Biopharma, Daewoong Therapeutics, Oncocross, and D&D Pharmatech. In 2021, Daewoong Pharmaceutical received approval for its gastroesophageal reflux disease treatment Fexclu, and in 2022, it successfully commercialized Envlo, its new SGLT-2 inhibitor class diabetes drug. In the first half of the year, the company's R&D investment amounted to KRW 104.8 billion, up 20.6% year-on-year. The company's increased R&D investment was driven by the adoption of promising technologies from biotech ventures. In March, the company paid KRW 6 billion to acquire the technology of an anti-cancer drug candidate that inhibits SOS1 from Cyrus Therapeutics and Kanaph Therapeutics. In Q2, the company paid KRW 3 billion in technology fees to biotech company J Ints Bio. J Ints Bio is a biotech company that develops new anti-cancer drugs. The company has also continued to increase clinical trial expenses for its new anti-cancer drug Leclaza. The company has been conducting Phase III clinical trials on Leclaza since 2020. Until the first half of this year, the company had invested KRW 111.2 billion in Leclaza’s Phase III trial. Dong-A ST invested KRW 80.3 billion in R&D in the first half of the year, up 49.5% year-on-year. The company’s clinical expenses for new drug development increased significantly. DA-4505, its immuno-oncology drug candidate, was approved for Phase 1/2a clinical trials in Korea in November last year. DA-4505 showed improved tumor suppression through preclinical studies compared to an AhR antagonist that is being developed by a multinational pharmaceutical company. Also, the company completed its Phase III trial for DA-8010, a treatment for overactive bladder, in Korea in May. However, DA-8010 did not show a statistically significant difference. SK Biopharm, HK Inno.N, Boryung, and Celltrion Pharm increased their R&D investments by more than 10% in the first half of the year compared to the same period last year. On the other hand, R&D investments by Handok, Hugel, GC Biopharma, Jeil Pharmaceutical, Daewon Pharmaceutical, Chong Kun Dang, and JW Pharmaceutical decreased compared to the same period last year. The increase in R&D investment amongst pharmaceutical companies that posted high sales was greater. The Top 10 sales companies - Samsung Biologics, Celltrion, Yuhan Corp, Hanmi Pharmaceutical, GC Biopharma, Chong Kun Dang, Daewoong Pharmaceutical, Boryung Pharmaceutical, HK Inno.N, and Dongkook Pharmaceutical - invested KRW 938.3 billion in R&D in the first half of the year, up 13.2% year-on-year. Of the Top 10 companies by revenue, the investment volume of 8 companies other than GC Biopharma and Chong Kun Dang increased year-on-year. It is analyzed that large pharmaceutical companies with experience in developing new drugs are actively investing in R&D to discover new candidates.
- Company
- Breast cancer drug Trodelvy receives DREC deliberations
- by Eo, Yun-Ho Aug 29, 2024 04:31am
- The ADC breast cancer drug Trodelvy has made a step toward insurance reimbursement after 9 months of wait. Gilead Sciences' triple-negative breast cancer (TNBC) drug Troldelvy, whose reimbursement request received 100,000 consents in a public petition, will be presented for deliberation to the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee on the 29th. The drug’s reimbursement application had remained pending for some time since its reimbursement standard was set by the Cancer Disease Review Committee in November last year. Therefore, the industry’s eye will be focused on the outcome of the committee review. The key issue will be the drug price, especially the ICER threshold value. Trodelvy is already listed in about 30 countries around the world. Trodelvy is already listed in about 30 countries around the world. Taiwan, which has a single-payer healthcare system similar to South Korea's, began reimbursing Trodelvy in February this year. The global rush to improve patient access to Trodelvy has been driven by the poor treatment environment for metastatic triple-negative breast cancer and the clinical value of Trodelvy. Triple-negative breast cancer is an aggressive form of breast cancer that recurs and metastasizes rapidly. Patients with metastatic triple-negative breast cancer who have metastasized despite treatment have a life expectancy of only a few months even with chemotherapy. However, chemotherapy has long been the standard of care due to the lack of targets that can effectively kill cancer cells. Trodelvy, the first Trop-2-targeted antibody-drug conjugate (ADC), is the only treatment for metastatic triple-negative breast cancer in the second-line or higher setting that has been shown to prolong survival compared to chemotherapy and has settled as the global standard of care since its introduction. Currently, major guidelines in the U.S. and Europe specify Troldelvy as the preferred agent for patients with previously treated metastatic triple-negative breast cancer. In a Phase III study, the overall survival of the chemotherapy arm was 6.9 months, compared to a nearly one-year survival. (11.8 months) in the Troldelvy arm, In addition, Troldelvy demonstrated an effect in controlling symptoms and pain caused by cancer and improving patients' quality of life by improving their overall health status. Trodelvy was awarded the highest possible score of 5 points on ESMO-MCBS, the European Society for Medical Oncology's (ESMO) scale used to rate the value of anticancer drugs. A score of 5 indicates that a drug is effective not only in prolonging patient survival but also in improving quality of life, and Troldelviy is the only treatment for metastatic triple-negative breast cancer to receive a score of 5 on ESMO-MCBS. In fact, the U.K. has detailed the rationale behind its assessment, stating that the state’s reimbursement decision was based on the severity of metastatic triple-negative breast cancer and the survival benefit of Troldelvy. Similar to Korea, the U.K. uses the incremental cost-effectiveness ratio (ICER) to evaluate new drugs for health insurance coverage. While the UK has one of the highest reimbursement barriers for new drugs, it applies flexible pharmacoeconomic evaluation criteria for innovative drugs used for serious conditions to improve patient access. In the UK, Troldelvy was granted preferential economic evaluation because it prolonged survival in terminally ill patients with less than 2 years life expectancy, whose population is even smaller than those of rare diseases. As a result, Trodelvy gained access with an ICER threshold that was approximately twice higher than that of general drugs. Meanwhile, Troldelvy has been the subject of a series of petitions this year, gathering more than 100,000 consents online. The Korean Alliance of Patients' Organizations also responded to the desperate pleas of patients and their caregivers when the petition was abandoned due to the expiration of the 21st National Assembly's term. In May, the organization submitted a letter directly to the Ministry of Health and Welfare requesting a prompt review of the reimbursement of drugs with high patient demand, including Trodelvy.
- Company
- "Switching of atopic dermatitis treatments not yet allowed"
- by Hwang, Byung-woo Aug 28, 2024 05:52am
- As new drug entries shift the market for atopic dermatitis, the public onion demands changes to reimbursement assessment, such as considering the comprehensive factors to use increased treatment options efficiently. Opinions have been suggested to consider factors related to patient quality of life, such as improving itchiness, in addition to the Eczema Area and Severity Index (EASI)-75 achievement rate, which is a typical criterion for evaluating the effectiveness of atopic dermatitis treatment. The view is that since switching between treatments is the major focus of the guidelines for atopic dermatitis treatment revised for the first time in 9 years and focus on switching between treatments, the Korean government must align with the global trend. During Pfizer Professor Ahn Ji Young, affiliated with the Department of Dermatology at the National Medical Center, and Professor Jang Yong Hyun, affiliated with the Department of Dermatology at Kyungpook National University, discussed the matter while sharing Korea's latest treatment trend of severe atopic dermatitis during the '2024 Pfizer Press University.' Regarding the necessity of switching between atopic dermatitis treatment, Ahn stressed the nature of the disease, which accompanies various factors. Ahn explained, "The treatment of atopic dermatitis is difficult because of genetic and allergic factors and accompanying disease," and "Patients experience improved and worsened symptoms. Therefore, the disease can progress to chronic disease, possibly leading to decreased patient quality of life and healthcare shopping." Ahn stresses the need to consider various factors for reimbursement assessment, such as itchiness, in addition to the previously defined EASI-75 score achievement. Ahn emphasized, "EASI-75 is a familiar indicator because reimbursement coverage is provided when patients with moderate-to-severe atopic dermatitis reach the score during the treatment. However, there are other important factors as well. Subjective symptoms such as itchiness must also be considered when evaluating reimbursement." To reflect on the current situation, experts emphasize switching between medications. Patient-optimized treatment options, depending on patient conditions, must become available. Jang stressed, "Biological agents and JAK inhibitors are currently used to treat atopic dermatitis. However, not all medications work for each patient," and "It is necessary to provide medication that suits patient's clinical outcomes, itchiness, and dermatological pathology. If it doesn't work, another medication must be allowed." (From left) Professor Ahn Ji Young, affiliated with the Department of Dermatology at the National Medical Center, and Professor Jang Yong Hyun, affiliated with the Department of Dermatology at Kyungpook National University. Academics suggest that the basis for switching between treatments is currently sufficient. While the approval criteria do not require the distinction between first-line treatment and second-line treatment, it has been suggested that a comprehensive factor such as the patient's condition must be considered when prescribing medications in clinical practices. Jang stated, "Academics stress the need for prescribing optimized medication to patients with severe symptoms after considering the patient's condition," and "Another medication must be allowed to be used when a patient does not benefit from one treatment, showing insufficient response." According to the pharmaceutical industry, unlike the initial request for authorization of switching therapy, the government may be more willing to bring changes now. For instance, the Health Insurance Review and Assessment Service (HIRA) has recently requested supplementary documents. Therefore, switching therapy may be considered based on academic's suggestion for patients with insufficient response. Jang said, "We were told there will be a meeting with the HIRA to discuss switching therapy."
- Company
- K-made Leclaza monotherapy to be showcased at global meeting
- by Son, Hyung-Min Aug 28, 2024 05:52am
- Korean biopharmaceutical companies continue to gain R&D achievements for targeted anti-cancer drugs. Companies have prepared to present their Korean-made anti-cancer drugs, including Leclaza and Rivoceranib at the 2024 World Conference on Lung Cancer, which will be held September 7-10 in San Diego, USA. Yuhan Pharmaceutical will present the clinical results comparing the efficacy of its proprietary Leclaza monotherapy, a non-small cell lung cancer (NSCLC) treatment, to Tagrisso. This month, Leclaza plus Rybrevant combination therapy passed the U.S. Food and Drug Administration (FDA) approval. A shift in the market for the first-line treatment of EGFR-positive NSCLC is expected if Leclaza monotherapy obtains better clinical results than Tagrisso monotherapy. HLB group and Jiangsu Hengrui Pharmaceuticals will present the clinical results of their targeted anti-cancer drug, Rivoceranib. The companies attempted to win FDA approval for Rivoceranib in combination with camrelizumab, a PD-1 immune checkpoint inhibitor. However, they received a complete response letter (CRL) request in May. HLB group will retry for approval by demonstrating effectiveness in various solid cancers, including liver cancer. Will present a follow-up study for the MARIPOSA trial, comparing Leclaza vs. Tagrisso monotherapies Treatments for NSCLC.According to industry sources on August 27th, the results of the study comparing the efficacy and safety profile of Leclaza monotherapy and Tagrisso monotherapy will be presented at WCLC 2024, to be held on September 4th. Leclaza and Tagrisso are treatments for targeting NSCLC harboring mutations of EGFR in exon 19 and exon 21 (L858R). Based on the Phase 3 MARIPOSA study results, disclosed last year, Leclaza plus Rybrevant combination therapy showed improvement in progression-free survival (PFS) compared to Tagrisso monotherapy. The study included patient treated with Leclaza monotherapy as a reference group. The results showed that the PFS for the Leclaza monotherapy group was 18.5 months, longer than 16.6 months for the Tagrisso monotherapy group. The company will present secondary results for the MARIPOSA study during the upcoming conference. The study is the first investigative analysis evaluating two third-generation EGFR-TKIs using randomization and a double-blind method. The study focused on 216 patients treated with Leclaza and 429 patients treated with Tagrisso. The primary endpoints included a blinded independent central view (BICR) PFS, duration of response (DOR), overall survival (OS), and safety. According to the disclosed abstract, BICR ORR for the Leclaza group was 88%, and for the Tagrisso group, it was 85%. However, the secondary analysis showed that Leclaza provided more clinical benefits than Tagrisso monotherapy in patients with biomarkers of high-risk disease, including TP53, circulating tumor DNA (ctDNA), and brain metastasis. In most solid cancers, patients with brain metastasis, TP53 mutations, and ctDNA detection tend to show poor prognosis. The median PFS for patients with a history of brain metastasis was 16.4 months for Leclaza, whereas Tagrisso's was 13.0 months. Among patients with detectable ctDNA, the median PFS for the Leclaza group was 18.4 months, and for the Tagrisso group, it was 14.8 months. Among patients with TP53 mutations, the Leclaza group had a median PFS of 14.6 months, which was longer than 12.9 months for the Tagrisso group. Two treatments had similar safety profiles, with moderate side effects between Grade 1 and Grade 2. Tagrisso group was more likely to have adverse reactions like diarrhea, thrombocytopenia, and reduced white blood cells. The Leclaza group was more likely to have rashes and paresthesia. The research team evaluated that "The efficacy and safety were comparable between Leclaza and Tagrisso. The results indicate that Leclaza can be a new treatment option for patients with EGFR-mutated advanced NSCLC and high-risk patients." Rivoceranib plus camrelizumab seen as a potential treatment for early lung cancer On September 8th, clinical trial results of the combination of the HLB group's Rivoceranib and Jiangsu Hengrui Pharmaceuticals' camrelizumab as perioperative neoadjuvant therapy. HLB group and Jiangsu Hengrui Pharmaceuticals, the developers of these two treatments, are evaluating the clinical efficacy of the combination of Rivoceranib, a vascular endothelial growth factor receptor-2 (VEGFR2) inhibitor, and camrelizumab, an immune checkpoint inhibitor. The current Phase 2 clinical trial evaluated the effectiveness and safety of Rivoceranib plus camrelizumab plus chemotherapy in patients with Stage 3 NSCLC. The patients received surgery after the combination therapy. The primary endpoint was the major pathological response (MPR) rate. The secondary endpoint included pathological complete response (pCR) rate, ORR, and safety. Based on the disclosed abstract, the combination of Rivoceranib plus camrelizumab plus chemotherapy as perioperative adjuvant therapy increased the rate of success for lung cancer surgery. The clinical results involving 29 patients showed that 19 patients had complete cancer removal. The combination therapy demonstrated 86.2% ORR, the percentage of patients with a confirmed objective response. The most common adverse reactions were white blood cell reduction (31.0%), thrombocytopenia (17.2%), rash (17.2%), and fatigue (13.8%). OS has not been reported due to incompleteness. The research group said, "The combination of Rivoceranib plus camrelizumab plus chemotherapy showed clinically meaningful anti-tumor activation and manageable safety without blood toxicity," and added, "It seems to be a potential treatment option for patients with resectable Stage 3 NSCLC." HLB group and Jiangsu Hengrui Pharmaceuticals plan to investigate potential in various fields, including liver cancer, gastric cancer, adrenocortical carcinoma, colorectal cancer, ovarian cancer, bile duct cancer, and esophagus cancer, in addition to early NSCLC.
- Company
- Challenges in the evolving lung cancer treatment landscape
- by Son, Hyung-Min Aug 28, 2024 05:51am
- The head-to-head battle between the epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer drugs (NSCLC) Leclaza and Tagrisso has carried on to competition of each drug's combination therapy regimens. This month, the U.S. Food and Drug Administration (FDA) approved Leclaza+Rybrevant as a first-line treatment for EGFR-positive NSCLC. This is the first time a targeted therapy plus targeted therapy combination has been approved. Tagrisso was approved in Korea and the U.S. this year after confirming its efficacy in combination with platinum-based chemotherapy. While attention is focused on whether combination therapies will find a place in the first-line treatment market for EGFR-mutated NSCLC, there is a consensus that the choice of a first-line treatment should be based on the patient's comprehensive needs, including side effects, frequency of hospital visits, and quality of life. Leclaza+Rybrevant combo improved overall survival compared with Tagrisso monotherapy NSCLC drug Leclaza Yuhan Corp and its partner Janssen have confirmed the efficacy of the combination of the Leclaza+Rybrevant combination therapy. Leclaza is a third-generation tyrosine kinase inhibitor (TKI) that targets exon 19, and exon 21 (L858R) in EGFR-positive NSCLC. Rybrevant is a targeted treatment option that targets the exon 20, MET mutation. The two companies successfully secured FDA approval for Leclaza+Rybrevant with the MARIPOSA Phase III trial. Specifically, the FDA approval was based on clinical results presented by Janssen at the European Society for Medical Oncology Annual Congress (ESMO 2023) last year. The results showed a median PFS of 23.7 months in the Leclaza+Rybrevantcombination arm, which was longer than the 18.5 months in the Leclaza monotherapy arm, and 16.6 months in the Tagrisso monotherapy arm. Leclaza+Rybrevant combination was associated with a 30% lower risk of disease progression and death than Tagrisso monotherapy. The interim OS analysis showed a trend favorable to Leclaza+Rybrevantover Tagrisso monotherapy. PFS2 results showed a 25% lower risk of disease progression or death in the Leclaza+Rybrevant combination arm compared with Tagrisso monotherapy arm. Tagrisso+platinum-based chemotherapy is approved in Korea and the U.S. NSCLC drug TagrissoTagrisso was approved in Korea based on the results of the FLAURA2 clinical trial. In May, the Ministry of Food and Drug Safety approved Tagrisso+platinum-based chemotherapy as a first-line treatment for EGFR-positive non-small-cell lung cancer. Tagrisso is a third-generation TKI developed by AstraZeneca. The Phase III FLAURA2 trial enrolled 557 patients with locally advanced or metastatic NSCLC who had received no prior systemic therapy and were positive for EGFR exon 19 deletion or exon 21 mutation. The study evaluated the efficacy and safety of Tagrisso combination therapy versus Tagrisso monotherapy. Results showed that Tagrisso+platinum-based chemotherapy reduced the risk of disease progression or death by 38% compared to Tagrisso monotherapy. Median investigator-assessed PFS was 25.5 months, an 8.8-month extension compared to 16.7 months with Tagrisso monotherapy. Median PFS by blinded independent central review (BICR) was 29.4 months, compared with 19.9 months in the Tagrisso monotherapy arm. Also, inpatients with the L858R mutation, Tagrisso+platinum-based chemotherapy showed a median PFS of 24.7 months, 10.8 months longer than the 13.9 months achieved in the Tagrisso monotherapy arm. Use of combination therapies boosts efficacy, but managing side effects remains key#EB Both Leclaza and Tagrisso have been granted additional regulatory approvals for their combination therapies, heating competition in the first-line treatment market for EGFR-mutated NSCLC. However, side effects management will be a key issue due to its combined use with new drugs. Rybrevant is an intravenous (IV) formulation that requires dosing once every three weeks. Adding an IV formulation to the existing oral formulation, Leclaza, could double the effectiveness but reduce dosing convenience. Janssen is also developing a subcutaneous (SC) formulation to significantly reduce dosing time and address concerns about infusion-related side effects. Recently published clinical results showed that the combination of the subcutaneous formulation of Rybrevant SC+Leclaza achieved similar outcomes to Rybrevant IV+Leclaza. At a median follow-up period of 7 months, the Leclaza+Rybrevant SC was non-inferior to Leclaza+Rybrevant IV. “The Leclaza+Rybrevant combination therapy has continued to generate positive data in brain metastases, L858R mutation, etc., and therefore can be a viable first-line treatment option for EGFR-mutated NSCLC,” said a professor of medical oncology at a university hospital. ”It is difficult to say that use of the combination therapy is good for all patients, as the use of combination therapy is associated with more side effects than using either drug as monotherapy. This is why prescribing Rybrevantto to older patients can be challenging.” “The use of the intravenous formulation of Rybrevantrequires much attention. While a subcutaneous formulation is currently in development, the existing intravenous formulation is associated with frequent rashes, paronychia, and infusion-related adverse events. When selecting a treatment, we should consider not just the efficacy but the patient's overall experience, including side effects, frequency of visits, and quality of life. So there will be an active debate about which patients will benefit most from the early use of the combination therapy.” Concerns also rise on the lack of later-line therapies following the use of Tagrisso+platinum-based chemotherapy The use of Tagrisso and platinum-based chemotherapy as first-line treatment may lead to a shortage of treatment options for patients who develop resistance. In the past, EGFR-mutated NSCLC targeted therapies have been used as follows: 3rd generation TKIs were used on patients who were confirmed to be T790M-positive during biopsy after using 1st and 2nd generation TKIs, and platinum-based chemotherapy (Alimta plus carboplatin/cisplatin) was used on T790M-negative patients. So if a 3rd-generation TKI monotherapy is used in the first line, platinum-based chemotherapy is often used as a second-line treatment as they cannot use 1st or 2nd-generation TKIs due to resistance. This suggests that the use of both Tagrisso and platinum-based chemotherapy in the first line may lead to a lack of later-line treatment options. After developing a resistance to the combination, only taxane drugs such as docetaxel and paclitaxel and immuno-oncology drugs targeting PD-L1 will remain as treatment options. “After failing treatment with EGFR-TKIs, we usually use platinum-based chemotherapy, Alimta+cisplatin/carboplatin,” says a professor of medical oncology at a university hospital. “Combining these drugs with 3rd-generation TKIs is certainly therapeutic. However, we should also consider the lack of later-line treatment options after the patient develops TKI resistance if we pull the next line of treatment in advance amid a shortage of treatment options.” “As 3rd-generation TKIs have settled as the first-line standard of care, the role of 1st- and 2nd-generation TKIs will continue to diminish, and it is likely that it will ultimately become a competition between 3rd-generation TKI monotherapy and combination therapy.”
- Company
- Value of new drugs should be evaluated based on efficacy
- by Hwang, Byung-woo Aug 27, 2024 05:50am
- 'Receiving recognization of the value of new drugs' is one of the key challenges for pharma and biotech companies. With the proportion of new drugs being introduced into Korea rising and the number of homegrown new drugs increasing, the need for a drug pricing policy that recognizes the value of these new drugs has been growing. Recognizing the value of new drugs can lead to a virtuous cycle that drives profits and motivation for subsequent research and development (R&D) in the industry. The 'Measure to Improve the Drug Pricing System to Reflect the Innovative Value of New Drugs' being discussed in this context has raised industry expectations, promising improved access to innovative new drugs and preferential treatment during pharmacoeconomic evaluations. However, as the system is being implemented for the first time, a gray zone where the interpretation of the regulations may differ does exist, raising concerns about the system’s practicality. In the long run, it will be interesting to see if the government's intention to reflect the innovative value of drugs translates into the real world. 'Innovation' condition added to the flexible ICER threshold... Will the threshold exceed KRW 55 million? On the 16th, the National Health Insurance Review and Assessment Service (HIRA) released the 'Detailed Evaluation Criteria for Drugs Subject to Negotiation, including New Drugs', which was revised based on the deliberations of the Drug Reimbursement Evaluation Committee (DREC). The revisions include ▲ establishment of ICER threshold elasticity evaluation criteria for drugs ▲ addition of severe diseases to the risk-sharing program ▲ omission of the Drug Evaluation Committee when expanding the coverage of risk-sharing drugs under 1.5 billion won ▲ and new conditions for submitting clinical evidence such as RWD and RWE when renewing risk-sharing programs. The most notable part is that the 'innovativeness of the new drug' standard had been specified in the criteria to flexibly evaluate the ICER (incremental cost-effectiveness ratio) threshold. Previously, the regulations referred to the use of an ICER threshold as “instead of using an explicit threshold, flexibly refer to the results of previous deliberations considering the severity of disease, social burden of disease, impact on quality of life, and innovativeness.” The government’s In the specified regulations, a new drug is now regarded as ‘innovative’ when it satisfies all of the following criteria: ▲ there is no substitutable or therapeutically equivalent product or treatment ▲ a significant clinical improvement, such as prolonged survival, is recognized in the final outcome, ▲ the new drug is approved by the MFDS under Article 35(4)(2) of the Pharmaceutical Affairs Act through expedited review or is recognized as equivalent by the committee. The question now is how much of the innovativeness of a new drug can be recognized. The industry believes that Enhertu's case will serve as one reference point. Although there is no clearly documented figure, it is generally accepted that the maximum ICER granted for reimbursement in Korea is KRW 50 million. And even the KRW 50 million threshold is known to have been granted very few times. The ICER threshold that the government had set for Enhertu is likely in the low KRW 50 million range. In other words, the drug was recognized as cost-effective despite exceeding the ICER threshold. In this regard, the pharmaceutical industry believes that the ICER threshold should be more flexibly applied given the effectiveness of each drug. A representative from a multinational pharmaceutical company said, “If a drug has such a good effect that it prolongs survival, the ICER is bound to increase because it is more costly to administer. This should be recognized to some extent, but if the flexible ICER range is in the KRW 55 million range, these good drugs will still have difficulty passing the threshold.” For example, the ICER threshold should be more reflective of the innovativeness of a new drug that comes out after decades of lack of treatment, or a treatment that has a much better hazard ratio during survival than existing treatments and improves survival by over twofold. KRPIA’s research on the R&D Investment Status of Global Pharmaceutical Companies Actual cases of flexible ICER application is key...KRPIA, “Flexible approach adopted by major countries should be considered” In particular, the industry predicts that actual cases of application will be important, especially given that many drugs that should be eligible for flexible application of the ICER threshold are awaiting evaluation. “I think it's positive that the government explicitly stated their intention to recognize innovation compared to how such a wording was non-existent regarding the application of the ICER thresholds,” said an industry representative. “There are about 8 drugs that would want to be recognized for their innovativeness, so we have high hopes for the new direction.” “However, if the government’s range of flexibility is adding a mere KRW 5 million to the current upper ICER limit of KRW 50 million, a big difference in perspective will remain. The company's efforts are important, but for real-world application, it remains to be seen whether the government will be willing to recognize an ICER threshold of an unconventional level, whether it is KRW 70 million or KRW 100 million.” The industry pointed out that there are many difficulties for drugs in passing pharmacoeconomic evaluations, such as the reduction in the price of alternative drugs due to various follow-up measures and DREC’s repeated requests for supplementary data until the desired price level is reached. It is unlikely that a small increase in ICER, which fluctuates greatly depending on the assumptions made, will be enough to get an innovative new drug approved quickly. The ICER threshold desired by multinational pharmaceutical companies is reflected in the 'KRPIA Policy Proposal for Improving Public Healthcare' that was released by the Korean Research-based Pharmaceutical Industry Association (KRPIA). In the 'Prospects for New Drug Access Policy' part of the policy proposal, KRPIA suggested that it is not easy to objectively evaluate the effectiveness and innovativeness of new drugs and that it is necessary to consider the flexible new drug evaluation methods of major countries. KRPIA New Drug Access Policy Outlook (in comparison with major countries) In the United Kingdom, “a broad and multi-layered ICER criterion is applied, which considers the disease characteristics, clinical utility, payment value, and societal needs,” and in Canada “ICER is a reference for drug pricing negotiations, and the decisive factor is the level of symptom improvement, and the price of the drug is set at the upper limit of the median drug price in 11 major countries. In Germany, “pharmaceutical companies may autonomously set the initial price and negotiate reimbursement and discount rates after 1 year by evaluating its innovativeness and usage,” and Japan also adopts a system that evaluates a drug’s innovativeness and assigns an adjusted price. In consideration of this global trend, KRPIA emphasized that Korea also needs to more flexibly view ICER, etc. to recognize the innovativeness of new drugs. While the pharmaceutical industry welcomes the inclusion of the 'new drug’s innovative value' and 'expanded coverage for severe and rare diseases' in the government's major policies, the industry is emphasizing the need to set a more specific direction. For example, Kay Bae, the Chair of KRPIA stated, “While the government's recognition of the value of new drugs is an encouraging achievement, the government needs to come up with more practical and concrete measures so that new drugs can be supplied to patients quickly and lead to a virtuous R&D cycle in Korea.” The amended system has measures in place that require observation, such as the submission of clinical evidence such as RWD and RWE when renewing RSA contracts. In the long run, the government and the pharmaceutical industry are eye-to-eye in that they believe in the need to recognize the 'innovativeness of new drugs'. Well begun is half done, and the pharmaceutical industry welcomes the government’s launch of the 'Measure to Improve the Drug Pricing System to Reflect the Innovative Value of New Drugs.’ How well the system is applied and supplemented will remain a future task.
- Company
- K-similars won record number of U.S.·Europe nods this year
- by Chon, Seung-Hyun Aug 27, 2024 05:50am
- Korean biopharmaceutical companies have launched the highest number of biosimilars in history this year in the United States and European markets. As Celltrion and Samsung Bioepis won approvals for six biosimilars over eight months in the world's largest market, they topped the previous record of five cases of five years ago. According to sources on August 27th, Celltrion's SteQyma, a biosimilar to Stelara for the treatment of autoimmune diseases, received marketing authorization from the European Commission (EC). It reached the final commercialization step two months after the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency's (EMA) committee issued a positive opinion recommending SteQyma approval at the end of June. Stelara (ustekinumab), developed by Janssen, is a treatment for autoimmune diseases prescribed for plaque psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis. It inhibits the activity of interleukin (IL)-12,23, a type of inflammatory cytokine involved in immune responses. Last year, the global market size for ustekinumab was estimated at US$ 20.4 billion (about KRW 26.5 trillion). The European market was estimated at US$3.1 billion (about KRW 4.0 trillion), taking 15% of the global market. After Samsung Bioepis won European approval for Pyzchiva, a biosimilar to Stelara, in April, Celltrion entered the European market as the second Korean company. In June, Samsung Bioepis obtained a marketing authorization for Pyzchiva. U.S. and European biosimilar approvals obtained by Korean biopharmaceutical companies: (2024) Celltrion won European approval for Omlyclo, a biosimilar to Xolair, and SteQyma, a biosimilar to Stelara in 2024. Samsung Bioepis won European approval for Pyzchiva, a biosimilar to Stelara, and U.S. FDA approval for Opuviz, a biosimilar to Eylea, Pyzchiva, and Epysqli, a biosimilar to Soliris in 2024 (source: respective companies and FSS). Celltrion and Samsung Bioepis won six approvals for biosimilars in the United States and Europe this year. It exceeded the previous record of five approvals in 2019, breaking the highest number of approvals in history. Celltrion received a marketing authroziation for Omlyclo, a biosimilar to Xolair, from the EC in May. Omlyclo is the first Xolair biosimilar to receive the European marketing authorization. Xolair is an antibody biosimilar used to treat allergic asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and chronic spontaneous urticaria (CSU). Samsung Bioepis won four biosimilar approvals this year in the United States and Europe this year, with three approvals in the United States and one in Europe. In April, Samsung Bioepis won European approval for Pyzchiva, a biosimilar to Stelara. Since May, Samsung Bioepis succeeded in obtaining biosimilar approvals from the U.S. FDA for the past three months. It received approval for Opuviz, a biosimilar to Eylea. Eylea, developed by Regeneron in the United States, is indicated for treating wet age-related macular degeneration (AMD). Eylea generated approximately KRW 13 trillion in global sales last year. After winning FDA approval for Pyzchiva in June, Samsung Bioepis obtained a marketing authorization for its orphan drug Epysqli. Epysqli, developed by Alexion in the United States, is a biosimilar to Soliris. Epysqli won FDA approval as a treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). In 2019, Celltrion and Samsung Bioepis received five biosimilar approvals in the United States and Europe. In November 2019, Celltrion received European approval for Remsima SC, a subcutaneous injection formulation of Remicade. Remsima SC is a biosimilar developed by Celltrion by changing the formulation of Remicade from intravenous (IV) to subcutaneous (SC) injection. In 2019, Samsung Bioepis received FDA approval for four biosimilars to Herceptin, Enbrel, Humira, and Lucentis. In January 2019, Ontruzant, a biosimilar to Herceptin, received a marketing authorization in the United States, followed by Eticovo and Hadlima, biosimilars to Enbrel and Humira, in April and July of the same year, respectively. Samsung Bioepis obtained FDA approval for its Byooviz, a biosimilar to Lucentis, in the United States in September 2021. Korean pharmaceutical companies have started actively targeting the global biosimilar market after Celltrion's Remsima received European marketing authorization under the title of 'The world's first antibody biosimilar' in August 2013. Since 2016, Korean biopharmaceutical companies continued to win new approvals yearly in the United States and Europe. Celltrion achieved success, obtaining eight approvals in Europe and six in the United States. The company also entered the European market for Remicade, Mabthera, Herceptin, Avastin, Humira, Xolair, and Stelara. In 2016, Celltrion's Inflectra, a biosimilar to Remicade, became the first to win FDA approval in the United States. In 2018, Truxima and Herzuma received FDA approvals. In September 2022, Celltrion obtained a marketing authorization for Vegzelma, a biosimilar to Avastin, from the FDA, and last year, Yuflyma, a biosimilar to Humira, also received FDA approval. In August last year, Celltrion received a marketing authorization for Zymfentra, a SC formulation of Remsima, as a novel drug. Samsung Bioepis won eight approvals in Europe and six in the United States. In January 2016, Samsung Bioepis began its global market expansion by obtaining approval for its Benepali, a biosimilar to the autoimmune disease treatment Enbrel, in Europe. Afterward, the company received European approval for biosimilars referencing Remicade, Herceptin, Humira, Avastin, Lucentis, Soliris, and Stelara. In April 2017, Samsung Bioepis received the first FDA approval in the United States for its Renflexis, a biosimilar to Remicade. After that, the company entered the U.S. market for biosimilars referencing Herceptin, Enbrel, Humira, Lucentis, Eyela, Stelara, and Soliris.
- Company
- More urothelial cancer drug options now available in KOR
- by Hwang, Byung-woo Aug 26, 2024 05:46am
- A number of immuno-oncology and antibody-drug conjugate (ADC) drugs are being granted approval as a first-line treatment of urothelial cancer, increasing the treatment options that had previously been dominated by platinum-based chemotherapy. Although they are not yet reimbursed and have yet to become mainstream treatment options, it is expected that the strategy for each line of treatment will change in the long term. (from left to right) Pic of Padcev, Keytruda, Opdivo Urothelial carcinoma starts in the epithelial cells lining the urinary tract and is the most common type of bladder cancer, accounting for 90% of all bladder cancer diagnoses. However, unlike other cancers like lung and breast cancer, where the standard of care changes quickly with the introduction of new drugs, UC has remained a barren area for decades, leaving a large unmet need for first-line treatment options. For the past 30 years, platinum-based chemotherapy has remained the first-line standard of care for UC. The introduction of Opdivo (nivolumab) has changed the situation. The drug was approved by the Ministry of Food and Drug Safety on the 17th as a first-line treatment for unresectable or metastatic urothelial carcinoma in combination with cisplatin and gemcitabine (GemCis). The approval was based on results from the Phase III CheckMate 901 trial in patients with unresectable or metastatic UC who had not received prior therapy. Results showed that at a median follow-up of 33.6 months, the primary endpoint, median OS (mOS), was 21.7 months with the use of the Opdivo+GemCis combination, which is significantly longer than the 18.9 months in the GemCis only arm, and reduced the risk of death by 22%. In particular, the CheckMate-901 pivotal trial, which is evaluating Opdivo in combination with Yervoy (ipilimumab) versus the standard of care, leaves room for further expansion of Opdivo’s indications. Keytruda (pembrolizumab) + Padcev (enfortumab vedotin), which received approval from the MFDS on March 2 5 following Opdivo’s approval, is also expected to rise as a first-line treatment option in UC. Highlighted as a novel combination of an immuno-oncology drug and an ADC, the Keytruda-Padcev combination gained approval as the first treatment to change the first-line treatment paradigm for UC in 30 years when the first data were presented at the European Society for Medical Oncology Annual Meeting (ESMO 2023) last year. The Phase III EV-302/KEYNOTE-A39 trial, which became the basis of its approval, the combination demonstrated a median progression-free survival (PFS) of 12.5 months over a median follow-up of 17.2 months, with a 55% reduction in the risk of disease progression and death compared to 6.3 months in the placebo arm. Dr. Jae-Lyun Lee, Professor of Medical Oncology at Seoul Asan Medical Center, said, “No other treatment in UC has shown this level of efficacy in the past 30 years. The more than twofold increase in progression-free survival is truly remarkable, which is why we have high hopes for the Keytruda-Padcev combination. Based on the clinical trial results, Padcev+Keytruda is expected to rise to the forefront among first-line treatment options on-site. Currently, Bavencio (avelumab) is reimbursed as a first-line maintenance therapy, but as new first-line treatment options become available, a change in the treatment regimen and later-line therapies is inevitable. However, Opdivo, which was added to existing first-line treatment options, and Keytruda+Padcev, an immuno-oncology drug, and an ADC combination, both are yet to be reimbursed in Korea, leaving the high cost an issue. Dr. Inho Kim, professor of Medical Oncology at St. Mary's Hospital in Seoul, said, “Drugs like Padcev have shown good results recently, and I think each drug has its pros and cons. Cost is also a consideration, and as we gain more prescription experience and accumulate more information on the patients’ conditions, we will be able to set guidelines for this.”