- LOGIN
- MemberShip
- 2025-12-22 17:07:45
- Company
- Chong Kun Dang speeds up dyslipidemia drug development
- by Son, Hyung Min Nov 05, 2024 05:46am
- Chong Kun Dang is accelerating the development of new drugs for dyslipidemia. The company has received approval to initiate a second global clinical trial in 10 years for its new drug candidate 'CKD-508' since the company began its research in 2014. According to industry sources on the 4th, Chong Kun Dang recently received approval from the U.S. Food and Drug Administration (FDA) to initiate a Phase 1 clinical trial on its dyslipidemia drug candidate, 'CKD-508'. Through the clinical trial, Chong Kun Dang plans to verify the safety and lipid-improving effect of CKD-508 and explore the optimal dose for its future Phase 2 trial. CKD-508 is a second-generation drug that overcomes the challenges of first-generation cholesteryl ester transfer protein (CETP) inhibitors, including limitations such as off-target effects (unexpected side effects caused by the drug), accumulation of fat cells, and poor stability. The drug candidate works by inhibiting the activity of CETP, which facilitates the transport of cholesteryl esters (CE) and triglycerides (TG) between fatty proteins in the blood, thereby lowering low-density lipoprotein cholesterol (LDL-C) levels and raising high-density cholesterol (HDL-C) levels. More than 6 years after initiating the CKD-508 study in 2014, Chong Kun Dang has now initiated global clinical trials. In June 2020, the company received approval from the UK's MHRA for a Phase 1 clinical trial of CKD-508 and is advancing its research and development. Preclinical results showed that CKD-508 significantly reduced LDL-C and LDL-C-containing apoprotein (Apo-B) and increased HDL-C in animal models with dyslipidemia. In addition, CKD-508 did not cause drug accumulation in adipose tissue or an increase in blood pressure, which were observed in clinical trials of anacetrapib and torcetrapib. Anacetrapib and torcetrapib were candidates for dyslipidemia developed by Merck and Pfizer, but the companies discontinued their development in 2017 due to safety concerns in the clinical trial stage. Chong Kun Dang believes that CKD-508 has the potential to be a breakthrough drug that could provide a new treatment option for patients with dyslipidemia that is not controlled by existing drugs such as statins. CKD-508 can be administered once weekly and may offer improved convenience over existing therapies. Along with CKD-508, Chong Kun Dang is also conducting 5 clinical trials in the synthetic drug category, including CKD-943 for uremic pruritus, CKD-950 (namodenoson) for hepatocellular carcinoma, CKD-951 for metabolic dysfunction-associated steatohepatitis (MASH), and CKD-510 for rare diseases. The company completed clinical trials for CKD-943 in the U.S. in 2015 and is currently in Phase III clinical trials. Chong Kun Dang has been developing CKD-943 since 2012 after signing a license agreement with Cara Therapeutics in the US for the exclusive development and sales of CKD-943 in Korea. CKD-950 recently entered Phase II clinical trials in Israel. In 2016, Chong Kun Dang entered into an exclusive domestic license agreement with Israel's Can-fite Biopharma for CKD-950, a novel liver cancer drug candidate. The company also acquired a domestic patent for CKD-950 in 2020. CKD-951, which is being developed as a treatment for MASH, has been recruiting patients since its IND approval in 2020. CKD-951 has a mechanism of action that improves inflammatory and fibrotic responses by acting on A3AR, which is overexpressed in liver inflammatory/fibrosis-promoting cells. CKD-510 was licensed out to Novartis in November of last year. CKD-510 is a histone deacetylase 6 (HDAC6) inhibitor that uses the company’s highly selective, non-hydroxamic acid platform technology. The drug candidate has demonstrated safety and tolerability in Phase I clinical trials in the U.S. and Europe.
- Company
- ‘Prevent MI recurrence through efficient LDL-C control'
- by Whang, byung-woo Nov 05, 2024 05:45am
- With the rise of metabolic diseases such as hypertension, diabetes, and hyperlipidemia increase in Korea, the prevalence of myocardial infarction and atherosclerotic cardiovascular diseases are also on the rise. The mortality rate of myocardial infarction is in the 20-30% range when it occurs for the first time, but the mortality rate increases sharply to 68-85% when it recurs, which is why efforts to prevent recurrence are being stressed now. In particular, one of the hot topics in treatment is how to manage LDL cholesterol, which is known to be an important factor in preventing the recurrence of atherosclerotic cardiovascular disease (ASCVD). In recent years, treatment options have become more diverse and multiple approaches have been proposed. Dr. Dong-Oh Kang, Professor of Cardiology and Cardiovascular Center at Korea University Guro Hospital, emphasized the need to effectively lower LDL cholesterol levels in high-risk patients. Dong-Oh Kang, Professor, Department of Cardiology, Korea University Guro Hospital “New drugs have changed the approach to LDL cholesterol management in high-risk patients” In severe cases of acute myocardial infarction, stenting or balloon angioplasty is performed to open up the blood vessel, as it is an emergency treatment for blocked blood vessels or low blood flow. However, these procedures are reactive, and it is important to use medications to prevent the same event from happening again. “It is important for patients who have had a myocardial infarction to use drugs to prevent further accumulation of atherosclerotic plaque and narrowing of the artery,” said Professor Kang. ”Lowering cholesterol to inhibit the progression of atherosclerotic plaque and preventing blood clots has become a key treatment.” This is why one of the most important topics in recent guidelines is to what level LDL cholesterol should be lowered in very-high-risk patients. Both domestic and international academic societies have proposed a strict management standard for patients with a history of atherosclerotic cardiovascular disease, with LDL cholesterol targets of less than 55 mg/dL and at least 50% lower than baseline. “The past guidelines suggested that LDL cholesterol levels could be as low as 100 mg/dL, but more potent drugs have come in a variety of combinations.” said Professor Kang, “As lowering LDL cholesterol levels has been shown to reduce the risk of atherosclerotic cardiovascular disease, even lower levels are now being recommended.” According to Kang, the suggested LDL cholesterol level for high-risk patients was less than 70 mg/dL in the 2010s, but by the late 2010s, patients with coronary artery disease or at very-high-risk were advised to lower their LDL cholesterol level to less than 55 mg/dL and at least 50% from baseline. In particular, the European guidelines suggest lowering LDL cholesterol levels to less than 40 mg/dL for patients with acute coronary syndrome who have had a recurrent event within the last 2 years. “Cardiologists who see patients with more severe acute myocardial infarction or patients undergoing procedures seem to be in agreement with the lower LDL cholesterol targets. However, some have concerns about lowering LDL cholesterol levels below 55 mg/dL or 70 mg/dL.” Diversification of treatment options, including PCSK9 inhibitors...“Strategy will change depending on reimbursement status” As Professor Kang noted, the lower LDL cholesterol target levels have been accompanied by the emergence of drugs that can effectively lower the levels to such targets. In the past, statins, which inhibit the synthesis of cholesterol in the liver, were the only drugs available to lower LDL cholesterol levels, but more strategies became available with the introduction of ezetimibe, which inhibits cholesterol absorption in the intestine, including statin and ezetimibe combinations. Then, the entry of monoclonal antibody drugs such as Repatha (evolocumab), a PCSK9 inhibitor, into the reimbursement system has transformed the clinical landscape. Currently, PCSK9 inhibitors are used in patients with myocardial infarction whose LDL cholesterol levels have not dropped sufficiently despite the use of high-intensity statins and ezetimibe. “It's important to monitor the dose escalation during initial therapy,” said Kang. “If LDL-C targets are not met, the dose should be increased and the patient reevaluated. If the maximum dose is not effective, a PCSK9 inhibitor such as Repatha, which has a faster LDL cholesterol lowering rate and is more potent, may be considered.” “In terms of Repatha’s effect, 19 out of 20 people will have lower LDL cholesterol level maintained, even at 30 mg/dL. In patients who had low LDL cholesterol, to begin with, we see reductions to less than 10 mg/dL.” In the long term, the introduction of oral bempedoic acid and injectable siRNA therapies is expected to further expand treatment options. In addition to access to treatments based on patient condition, Professor Kang predicts that treatment approaches will change based on the drug’s reimbursement status. “As more effective treatments will continue to be developed, we expect more and more combination options to emerge, and it is necessary to prescribe them considering the patient's condition and the characteristics of each drug,” said Kang. ”Since there are various drugs, their use will likely be determined by how reimbursement is applied in high-risk patients.” In addition to secondary prevention, Kang emphasized the need for policy promotion to screen and manage patients before they become high-risk. “Even though people are sufficiently screened and informed about their risk factors through health screenings, they often overlook them and look back in retrospect after they become ill. It is necessary to always receive screening and make efforts to properly treat or improve lifestyle habits from the primary prevention stage.”
- Company
- New oHCM drug 'Camzyos' nearing approval for reimb in KOR
- by Eo, Yun-Ho Nov 05, 2024 05:45am
- Product photo of Camzyos. 'Camzyos,' a new drug to treat obstructive hypertrophic cardiomyopathy (oHCM), is nearing 90% approval for insurance reimbursement listing. Sources said that BMS Pharmaceutical Korea and the National Health Insurance Service (NHIS) concluded drug pricing negotiations for Camzyos (mavacamten), a new drug for obstructive hypertrophic cardiomyopathy (oHCM). The drug had previously faced a delay in the decision, but the company quickly reached an agreement this time. As a result, Camzyos is likely to be listed within this year. This drug received a re-assessment status during the Drug Reimbursement Evaluation Committee (DREC) review of the Health Insurance Review and Assessment Service (HIRA). After that, it passed the DREC review and entered a drug pricing negotiation in August, but the drug did not receive a decision during the negotiation period (60 days). Camzyos is the only drug that selectively inhibits cardiac myosin-actin cross-bridge formation, which is the cause of oHCM. Camzyos' mechanism involves dissociating myosin from actin, relaxing overstimulated heart muscle, and thereby improving left ventricular outflow tract (LVOT) structure and LVOT outflow obstruction. Due to the lack of available treatments for oHCM for a long time, off-label medications have been used to manage symptoms. After Camzyos launched, the European Society of Cardiology (ESC) updated its guidelines for managing cardiomyopathy for the first time in about nine years. Previously, the guidelines for HCM were based on evidence limited to small-scale monitoring data, retrospective analysis results, and consensus opinion. However, Camzyos has completely changed this situation. Two large-scale, phase 3 clinical trials conducted as randomized controlled trial (RCT) have confirmed the significant effect of Camzyos. Consequently, ESC guidelines recommend Camzyos with the highest evidence level A for the first time in treatment options. American College of Cardiology (ACC) and the American Heart Association (AHA) are preparing to update their guidelines. Furthermore, based on this phase 3 trial evidence, the U.S. FDA granted Camzyos Breakthrough Therapy Designation (BTD) and approval. Meanwhile, the efficacy of Camzyos was demonstrated through Phase 3 EXPLORER-HCM trials. In this trial, Camzyos improved primary endpoints, which were the patient’s symptoms (NYHA classification) and exercise capacity measured with peak oxygen uptake (pVO2), more than twofold compared to the placebo. 20% of the patients treated with Caymzyos met the NYHA classification and pVO2 improvements. It also reduced the LVOT outflow obstruction index by four-fold after exercise. 7 out of 10 patients who received Camzyos treatment had improved indexes and ended up not considering surgery, and they maintained the effects for 30 weeks.
- Company
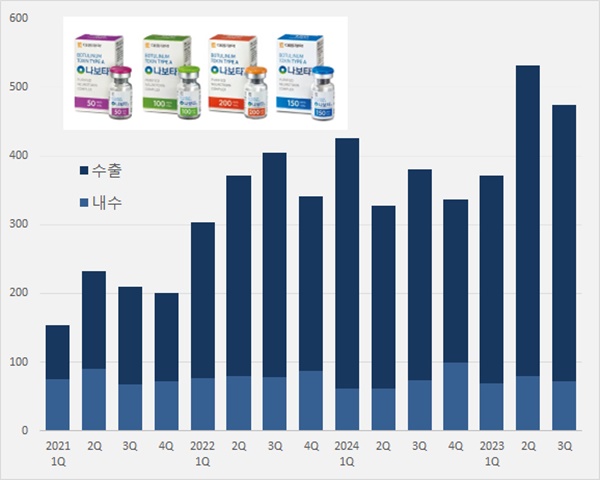
- 'Nabota' generated KRW 115.8B over 9 mths in foreign mkt
- by Chon, Seung-Hyun Nov 05, 2024 05:45am
- Daewoong's Nabota, which contains botulinum toxin, is expanding its presence in the foreign market. Its export amount surpassed KRW 100 billion up to Q3 2024. Over 80% of the overall sales were accounted for by sales generated in the foreign market. It is a cash cow export product. According to Daewoong on November 2, Nabota generated sales of KRW 47.5 billion in Q3, up 25.0% from KRW 38 billion Year-over-Year (YoY). Nabota's domestic sales amounted to KRW 7.2 billion in Q3, down 2.7% from the previous year. The export amount increased to KRW 40.3 billion, up 31.7%. Nabota's cumulative sales were KRW 137.8 billion in Q3, an increase of 21.6% from the previous year. Quarterly Nabota sales trend (unit: KRW 100 million, source: Daewoong). The export sales of Nabota have significantly increased due to its established credibility in the United States, following Daewoong's settlement with Medytox in a lawsuit for a stolen strain in 2019. In February 2021, Medytox settled a tri-party agreement with Daewoong's U.S. partnering companies, Evolus and AbbVie, for the sales of Nabota (marketed as Jeuveau in the United States) in the United States. Medytox and AbbVie will grant U.S. sales·marketing rights of Jeuveau to Evolus, and they will receive royalty payments. Nabota recorded export sales of KRW 6.4 billion in Q3 2020, an increase of over six-fold in four years. Nabota recorded cumulative export sales of KRW 115.8 billion until Q3 2024, up 23.7% Year-over-Year (YoY). In just 9 months, it nearly reached last year's export sales of KRW 117.4 billion. Export sales account for 84.0% of Nabota sales in Q3. The sales in the foreign market are substantial with continued exports between Q1 and Q3 this year at around 80%. Nabota ranks second in the cosmetic market for botulinum toxin in the United States, with a 13% market share. "In the first half of the year, Nabota's U.S. cosmetic sales surpassed Ipsen France's Dysport," the company said. "Nabota has recorded the highest growth rate among the cosmetic botulinum toxin brands. We continue establishing it as an outstanding brand with the highest quality overseas." In June, Evolus officially launched Nabota in Spain, expanding into the European market. Nabota is marketed as Nuceiva in Europe, and it has been launched in Spain, the UK, Germany, Austria, and Italy, continuing to expand to European countries in addition to the United States. In June, Nabota received marketing authorization from Argentina's National Administration of Drugs, Food and Medical Technology (ANMAT), and in September, it was launched in Malaysia. Nabota received marketing authorization from 68 countries worldwide, and the company has signed partnership agreements with 80 countries. Nabota is nearing its launch into the treatment market, which accounts for half of the global botulinum toxin market. Nabota's U.S. partnering company, iON Pharma, for the treatment indication is speeding up clinical trials for indications such as ▲chronic migraine ▲myotonia ▲gastroparalysis ▲PTSD. Daewoong has begun expanding its product plant ahead of increased demand for Nabota. The company invested KRW 101.4 billion in Hyangnam plant in Hwaseong, Gyeonggi Province to build the third plant with a yearly production size of 13 million vials. The third plant construction will commence in the first half of this year. When the third plant is completed, Daewoong will secure up to 18 million vials production capacity, including a yearly 5 million vials production from plants 1 and 2. "Nabota is a high-purity toxin manufactured using a patented and proprietary 'HI-PURETM Technology.' It provides an advantage of fast and accurate effects and safety towards drug tolerance," said Daewoong representative.
- Company
- 'SGLT2·DPP4' comb market records robust growth
- by Kim, Jin-Gu Nov 04, 2024 05:48am
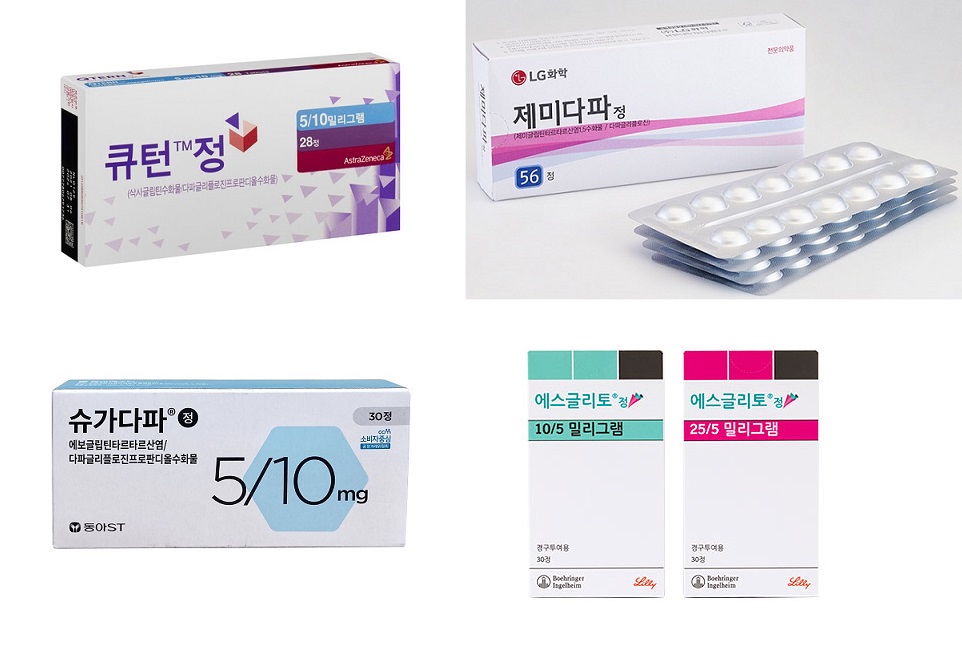
- Product photos of Qtern, Esgliteo, Esgliteo, and Sugadapa (clockwise from upper left). The market for combination drugs containing SGLT-2 inhibitor and DPP-4 inhibitor for treating type 2 diabetes is showing rapid growth. The industry expects the market size to expand by over KRW 30 billion this year. Products containing one or more original active ingredients are leading the market growth. Boehringer Ingelheim's 'Esgliteo' ranked top in prescription sales with cumulative sales of KRW 8.6 billion this year. It was followed by LG Chem's 'Zemidapa,' with KRW 6.1 billion, and AstraZeneca Korea's Qtern, with KRW 3.5 billion. 'SGLT2i+DPP4i combination drugs' generated KRW 9.5B in Q3…Will it surpass yearly KRW 30B? According to a pharmaceutical market research firm UBIST on October 2, two-drug combination drugs with SGLT-2 inhibitor and DPP-4 inhibitor generated KRW 9.5 billion in net sales in Q3 2024. The sales rose significantly over three-fold compared to KRW 2.8 billion of Q3 2023. This market was established with expanded reimbursement for diabetes combination therapy since April 2023. The government approved reimbursement of SGLT-2 inhibitor and DPP-4 inhibitor combination. After that, combination drugs containing different combinations were launched. In the same month, the patent of SLGT-2 inhibitor, 'Forxiga (dapagliflozin),' expired. Companies with original products with DPP-4 inhibitor have started to launch combination drugs with dapagliflozin class. In September, the DPP-4 inhibitor 'Januvia (sitagliptin)' patent expired. After that, generic companies without original active ingredients jumped into the competition. Quarterly prescription market size of SGLT2i+DPP4i combination drugs (unit: KRW 100 million, source: UBIST). Earlier in the launch, two-drug combination drugs generated below-expected sales. In 2023, the sales amounted to KRW 700 million in Q2 and KRW 2.8 billion in Q3. According to an analysis, the sales rose from Q4. It recorded KRW 5.3 billion in Q4 2023. This year, the sales significantly rose to KRW 7 billion in Q1, 8.2 billion in Q2, and 9.5 billion in Q3. The pharmaceutical industry expects sales to surpass KRW 10 billion for Q4 2024. The cumulative prescription sales in Q3 amounted to KRW 24.7 billion. This year's market size is expected to expand to over KRW 30 billion. Combination drugs with original products such as Esgliteo·Zemidapa show strong growth By products, products with original active ingredient are showing strong growth regardless of Korean pharmaceutical companies or global pharmaceutical companies. Boehringer Ingelheim's 'Esgliteo' generated the highest prescription sales in Q3 2024 with cumulative sales of KRW 8.6 billion. Last year, Boehringer Ingelheim launched two-drug combination drugs with its in-house originals, Jardiance (empagliflozin) and Tradjenta (linagliptin). It was followed by LG Chem's 'Zemidapa' with cumulative prescription sales of KRW 6.1 billion. Zemidapa contains LG-Chem's proprietary Zemiglo (gemigliptin) and dapagliflozin. The company launched this product after Forxiga's patent expired in April 2023. The next in sales is Astra Zeneca's 'Qtern' with KRW 3.5 billion. AstraZeneca combined its proprietary original Forxiga and Onglyza (saxagliptin). In South Korea, Ildong Pharmaceutical is responsible for the distribution of Qtern. AstraZeneca also has 'Sidapvia' with a different combination. Sidapvia's cumulative prescription sales are KRW 1.2 billion. It is a combination drug with dapagliflozin·sitagliptin. AstraZeneca collaborated with SK Chemicals to develop this drug and launched it in the market around the time of Januvia's patent expiration in September 2023. In South Korea, SK Chemicals is responsible for domestic production, and HK inno.N distributes the drug. The next drugs in rank are Chong Kun Dang's 'Exiglu-S' with KRW 1.1 billion, and Dong-A ST's 'Sugadapa' with KRW 1 billion. Chong Kun Dang launched Exiglu-S with a combination of Januvia, acquired from MDS, and dapagliflozin. Dong-A ST launched Sugadapa with a combination of its proprietary DPP-4 class Suganon (evogliptin) and dapagliflozin. The remaining 50 companies have generated cumulative prescription sales below KRW 500 billion up to Q3 2024. Most of these companies compete with products with dapagliflozin+sitagliptin. However, analysis suggests that their sales are below expected because they have entered the market later than other combination drugs with originals over five months, and many products were launched around the same time.
- Company
- Will the polycythemia vera drug Besremi be reimb in KOR?
- by Eo, Yun-Ho Nov 04, 2024 05:48am
- Whether PharmaEssentia Korea’s new drug for polycythemia vera, ‘BESREMi,’ will be listed with reimbursement in Korea is gaining attention. The drug was approved for hydroxyurea-refractory or intolerant polycythemia vera in March last year but failed to overcome the CDDC barrier in July of the same year. At that time, the CDDC determined that there was insufficient evidence to determine the clinical utility of BESREMi as a second-line treatment. In response, PharmaEssentia resubmitted its application for reimbursement in March after adding domestic clinical data on BESREMi and supplementing the evidence on the drug’s efficacy in second-line therapy. As 50,000 people signed a petition for improved access to BESREMi to the National Assembly in February, whether the company will succeed in receiving reimbursement this time is gaining close attention. BESREMi is a next-generation interferon treatment that selectively removes JAK2 mutations that cause polycythemia vera. I It was developed to improve the purity and tolerability of existing interferons so that it can be administered every two weeks for the first 1.5 years and every four weeks thereafter. It is currently recommended for the treatment of PV in the National Comprehensive Cancer Network (NCCN) and European Leukemia Network (ELN) guidelines, regardless of prior treatment history. Polycythemia vera is a rare blood disorder where a somatic cell mutation in the bone marrow abnormally activates bone marrow function and produces excessive red blood cells. According to HIRA data, about 5,000 patients are affected with PV in Korea, and hydroxyurea is mainly used for the majority of patients. However, as the current reimbursed drugs are not curative and there are no new alternatives for patients who fail hydroxyurea treatment, there remains a high unmet need for the disease.
- Company
- K-Bios globally present immuno-oncology drugs
- by Son, Hyung Min Nov 04, 2024 05:48am
- The development achievements of the domestic pharmaceutical bio industry's immuno-oncology drugs will be presented at an overseas conference. Hanmi Pharmaceutical, GC Cell, Abion Bio, ST Cube, and Y-Biologics, among others, have completed preparations to emerge into the international stage by disclosing positive clinical trial results. In particular, some companies are conducting clinical trials for combination therapies that utilize their approved immuno-oncology and targeted therapy drugs. They are aiming to increase the chances of commercialization by combining the drugs with verified therapies. Bispecific antibodies emerge as a global R&D trend, Korean companies also make a bid into the industry According to industry sources on the 4th, The Society for Immunotherapy of Cancer’s annual meeting (SITC 2024) will be held in Houston, U.S., from April 6 to 10. STIC is the world's largest immuno-oncology society with more than 5,000 industry professionals from more than 70 countries around the world. Hanmi Pharmaceutical will present the clinical trial results for its bispecific antibody, which has emerged as a global R&D trend. The company is conducting a Phase I clinical trial on BH3120, a bispecific immuno-oncology drug candidate. BH3120 simultaneously targets PD-L1 and 4-1BB. Bispecific antibodies are drugs that can bind to two different antigens simultaneously, or to two different epitopes on the same antigen. Such antibodies that simultaneously target various biomarkers have the advantage of allowing BBB penetration through targeted binding to receptors on the surface of the blood-brain barrier. In particular, anticancer drugs need to penetrate the BBB to increase drug permeability. Recently, a growing number of companies have developed multispecific antibodies by combining antibodies that bind to antigens that regulate the activity of immune cells and antibodies that bind to specific antigens on tumor cells. In clinical trials, BH3120 showed a decoupling of immune activity between the tumor microenvironment and normal tissue, confirming its safety. In addition to immuno-oncology drugs, Hanmi Pharmaceutical and Beijing Hanmi Pharm are also exploring the possibility of combining the drug with other anticancer drugs. BH3120 incorporates Hanmi’s platform technology, “Pentambody.” This is a next-generation bispecific antibody platform technology that activates immune cells while attacking only target cancer cells. Since 4-1BB is activated only in immune cells surrounding cancer cells expressing PD-L1, BH3120 minimizes the toxic side effects of 4-1BB and has long-term anti-cancer effects that prevent recurrence. Y-Biologics will present clinical results from AR092, one of its T-cell bispecific antibody pipeline. AR092 is a drug candidate generated from Y-Biologics’s proprietary next-generation T-cell bispecific antibody platform, “ALiCE.” Y-Biologics confirmed the potent anti-cancer effect and low toxicity of AR092 against solid tumors through a preclinical trial. SITC 2023(Source= SITC). Developing immuno-oncology drugs targeting novel mechanisms of actions Cell therapies and immuno-oncology drugs targeting new mechanisms of action will also be introduced at SITC 2024. G-Cell will present preclinical results of its NK cell therapy candidate “GCC4001” in combination with Merck's EGFR antibody therapy Erbitux. In preclinical studies, GCC4001+Erbitux demonstrated approximately 2 times the anti-tumor activity of Erbitux monotherapy. GC Cell believes that the combination may represent a novel treatment alternative for recurrent or metastatic head and neck cancer. GC Cell will also present unique findings on its proprietary NK cell culture technique based on eHuT-78 CDV feeder cells. ST Cube will present clinical results on its “nelmastobart,” which targets a novel biomarker, BTN1A1. BTN1A1 is a protein that regulates the immune response to cancer cells by inhibiting the activity of T-cells. This biomarker is not expressed in normal cells, but is strongly expressed in cancer cells and is mutually exclusive with PD-L1. By targeting BTN1A1, ST Cube is developing an immuno-oncology drug that may represent a new treatment option in refractory cancers. Currently, ST Cubde is conducting Phase Ib/II clinical trials in the U.S. and South Korea for nelmastobart in combination with paclitaxel in patients with relapsed or refractory extensive-stage small cell lung cancer (ES-SCLC). The company is also exploring the potential of nelmastobart in combination with capecitabine for the treatment of Stage III or higher metastatic colorectal cancer. Abion Bio is presenting preclinical results on “ABN202,” an antibody-cytokine fusion protein (ACFP) that fuses an interferon-beta variant to a tumor-targeting antibody. ACFP is a novel drug candidate that is expected to address the systemic toxicity of interferon-beta thereby offering a strong safety profile. The company will present nonclinical data confirming immuno-oncology efficacy in immunosuppressive solid tumor mouse models. Abion Bio is also exploring the potential for combination therapy with existing immuno-oncology agents.
- Company
- Reimb of Vocabria+Rekambys for HIV gains attention
- by Eo, Yun-Ho Nov 04, 2024 05:48am
- The industry’s eyes are on whether the long-acting HIV combination therapy ‘Vocabria+Rekambys’ will be reimbursed by the end of the year in Korea. According to industry sources, GSK Korea and Janssen Korea have completed the pharmacoeconomic evaluation of their HIV drugs Vocabria (cabotegravir) and Rekambys (rilpivirine) combination therapy and are awaiting the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee’s review. It remains to be seen whether the drug will be reviewed by DREC by the end of the year and progress onward. As it has been more than 2 years since the drug’s domestic approval, the industry’s eyes are on whether it will be reimbursed this time. The MFDS previously approved the two drugs in February 2022 as a combination therapy for the treatment of HIV-1 infection in adult patients who are virologically suppressed, have no history of virologic failure, and have no known or suspected resistance to either cabotegravir or rilpivirine. The Vocabria+Rekambys combination was approved in Korea as a once a month or once-every-two-month injection regimen. The advantage of the combination is convenience. Previous HIV treatments required patients to take tablet formulations once a day, so every day, but with the approval of the two injectables, patients will be able to take intramuscular injections once a month or every other month, reducing the frequency and increasing patient satisfaction. The two drugs were originally developed as oral formulations and then were developed as injectables. The long-acting injectables do not cure HIV infection, but they work by targeting white blood cells to help lower and maintain levels of the AIDS virus. The combination was approved in Europe in December 2020 after demonstrating efficacy and safety in clinical trials in groups receiving the combination once every 4 weeks or once every 8 weeks. In the clinical trials, the most frequent adverse events observed in the Vocabria+Rekambys arm were injection site reactions, headache, pyrexia, nausea, fatigue, asthenia, and myasthenia. It remains to be seen if the benefit of convenience of the combination therapy will be accepted by the health authorities and be listed for reimbursement.
- Company
- New AML drug Vyxeos can be prescribed at Big 5 hospitals
- by Eo, Yun-Ho Nov 01, 2024 05:51am
- The new acute myeloid leukemia drug ‘Vyxeos’ may now be prescribed at general hospitals in Korea. According to industry sources, Vyxeos (daunorubicin+ cytarabine), a treatment for adults with acute myeloid leukemia AML, has passed the drug committees (DCs) of the ‘Big 5’ tertiary hospitals in Korea including the Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary’s hospital, Sinchon Severance hospital, several medical institutions in Korea including the National Cancer Center, Samsung Medical Center, Seoul National University Hospital, and Asan Medical Center, and Sinchon Severance Hospital. The company also made progress in the drug reimbursement discussion. Vyxeos passed the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee review in August. If the company succeeds in completing pricing negotiations with the National Health Insurance Service and the drug is listed for reimbursement, it is expected to quickly lead to actual prescriptions. Vyxeos was developed by an Ireland-based global pharmaceutical company Jazz Pharmaceuticals headquartered in Ireland, and Handok owns the exclusive rights to its sales in Korea. Treatment-related adult AML and AML with myelodysplasia-related changes have a poor prognosis and are associated with lower remission rates and shorter overall survival (OS) when treated with intensive chemotherapy compared to other AML. Intensive chemotherapy, the 7+3 regimen of cytarabine and daunorubicin, has remained the standard therapy for over 50 years since its introduction in the 1970s. This is why an unmet need existed in the field for some time. Vyxeos is a liposomal formulation of a fixed combination of daunorubicin and cytarabine in a 1:5 molar ratio. Vyxeos liposomes accumulate and persist at a higher concentration in the bone marrow, where they are preferentially taken up intact by leukemia cells, maximizing synergistic antitumor activity. In a Phase III trial that demonstrated the efficacy of Vyxeos, the median overall survival of patients with t-AML or AMLM-MRC who were treated with Vyxeos was 9.6 months, which was longer compared with the 6 months in patients that received the 7+3 arm. Also, 48% of the Vyxeos-treated patients achieved complete remission (CR) and complete remission with incomplete neutrophil or platelet recovery (CRi), which was higher than the 33% in the 7+3 arm. The safety profile in both arms was similar.
- Company
- Novartis’s operating income grew 123% in Q3 with Entresto
- by Son, Hyung Min Nov 01, 2024 05:50am
- The Swiss global pharmaceutical giant Novartis' sales increased slightly compared to the previous year. Novartis showed even sales growth in various therapeutic areas, including cardiovascular, anticancer, and immunosuppressive agents. According to industry sources, Novartis reported a revenue of USD 12.823 billion in Q3 last year, up 10% YoY. The company’s operating income was approximately USD 3.627 billion, a 123% increase YoY. The largest revenue generator in Q3 was the heart failure drug Entresto. Entresto generated USD 1.865 billion in revenue, up 26% YoY. Entresto is an angiotensin receptor-neprilysin inhibitor (ARNI) class heart failure drug and is the only innovative drug that works directly on the heart. Its strength lies in its ability to be used as a first-line treatment for heart failure in combination with other medicines, including SGLT-2 inhibitors. Entresto’s sales have continuously increased with additional indications. Entresto was initially approved for use in heart failure patients with reduced ejection fraction, defined as a left ventricular ejection fraction of 40% or less. Through clinical trials, Novartis has been successful in expanding the indication of Entresto to patients with heart failure with preserved ejection fraction. Novartis' biologic treatment Cosentyx generated the second-largest revenue. Cosentyx generated sales of USD 1.693 billion, up 28% YoY. Cosentyx, which targets anti-interleukin (IL)-17, is effective in a wide range of inflammatory diseases and has emerged as a competitor to tumor necrosis factor alpha (TNF-α) inhibitors such as Humira and Remicade. The highest sales growth rate was seen in the PCSK9 inhibitor Leqvio. Leqvio’s sales totaled $198 million, a 119% year-over-year increase. Leqvio is a first-in-class siRNA drug approved in Korea, the U.S., and Europe. Leqvio uses naturally occurring siRNA to reduce LDL-C in the blood by inhibiting the production of the PCSK9 protein, which raises LDL-cholesterol (LDL-C). Leqvio has been shown to reduce LDL-C by up to 57% with twice-yearly dosing. The rise in sales of the prostate cancer drug Pluvicto was also notable. The company reported Q3 sales of USD 386 million, a 50% YoY increase. Pluvicto is a radiopharmaceutical Notavis acquired through the acquisition of US-based Endocytein in 2018. Sales of Pluvicto, which was introduced to the market in 2022, surpassed USD 200 million (KRW 260 billion) the same year. Since then, it has continued to grow, reaching nearly USD 1 billion (about KRW 1.3 trillion) in sales last year. Sales of the breast cancer drug Kisqali increased 43% year-on-year. Kisqali is a CDK4/6 inhibitor that targets HR+/HER2- breast cancer. The fact that it was the first CDK4/6 inhibitor to be prescribed to premenopausal women contributed to its rapid market growth. According to the National Comprehensive Cancer Network (NCCN) guidelines, Kisqali is recommended as a Category 1 in the first-line treatment of HR+/HER2- peri-menopausal breast cancer patients. In addition to its strong cardiovascular, oncology, and immunosuppressive pipeline, Novartis is also actively pursuing radiopharmaceuticals and cell therapies to reinforce its portfolio. Earlier this year, Novartis acquired the Dutch immunotherapy company Calypso Biotech for USD 425 million (about KRW 560 billion). Calypso owns a cell therapy candidate, ‘CALY-002,’ with other monoclonal antibodies in development for various immune disorders.