- LOGIN
- MemberShip
- 2025-12-22 11:32:05
- Company
- Only half of the multiple myeloma drugs reimb in KOR
- by Eo, Yun-Ho Jan 20, 2025 05:54am
- Despite the increased number of treatment options, patient access to those multiple myeloma drug options has not changed much. Multiple myeloma remains an incurable disease, but in the past, survival rates were very low due to limited treatment options. In recent years, however, innovative treatment options such as monoclonal antibodies, CAR-T therapies, and bispecific antibodies have diversified the treatment options, improving survival. In fact, over the past 20 years, the five-year survival rate for multiple myeloma patients has increased from 29.8% in 2001-2005 to about 50.1% in 2017-2021. However, this is still less than the 60% survival rate found in developed countries such as the United States, and limitation in access to care is regarded as the major contributing factor. Only half of the guideline-recommended drugs are reimbursed in Korea In Korea, only 13 (52%) of the 22 drugs recommended in the NCCN guidelines for multiple myeloma are covered by reimbursement (based on the NCCN guidelines 2024 v2). For example, Darzalex (daratumumab) was approved in 2019 as a first-line combination therapy for multiple myeloma but was only granted reimbursement as a fourth-line monotherapy in Korea. In October last year, 5 years since the Drug Reimbursement Evaluation Committee recognized the appropriateness of expanding Darzalex’s reimbursement coverage under the Risk Sharing Agreement (RSA). Also, Xpovio (selinexor) was granted reimbursement in July 2024, after 4 reimbursement attempts after its approval in 2021. Burden of proving cost-effectiveness for rare cancers such as multiple myeloma One of the reasons why it takes longer to reimburse the crucial multiple myeloma drugs and hinders access is that multiple myeloma is a rare cancer, which renders it more difficult to prove cost-effectiveness than other cancer drugs. In order to apply for reimbursement of high-priced anticancer drugs, the companies must submit data that demonstrates the drug’s cost-effectiveness, as per the guidelines for pharmacoeconomic evaluations. In particular, due to the rising financial expense spent on anticancer drugs in recent years, the government has been setting higher standards for the submitted data to demonstrate the improvement in the effectiveness of new drugs over existing drugs. However, multiple myeloma is similar to rare diseases in that it has a limited number of patients that can enroll in clinical trials, and it is difficult to set a comparator drug. In the case of anticancer and rare disease drugs, clinical trials often have a single-arm design or are limited to Phase II studies, which introduces uncertainties and challenges in the reimbursement review process. Add to this, the number of new treatment options has been growing. Recently, bispecific antibodies, which are regarded as the next-generation biotechnology, have been approved and released for multiple myeloma. Bispecific antibody treatments are immune cell therapies that consist of two monoclonal antibodies that recognize the target antigens of multiple myeloma and T cells. Bispecific IgG2 kappa antibodies, which are composed of two monoclonal antibodies that recognize the target antigens of multiple myeloma, B-cell maturation antigen (BCMA) and CD3 antigen, respectively, are common and are a novel treatment that directly targets cytotoxic T cells to BCMA-expressing multiple myeloma cells. Despite their high clinical efficacy, the bispecific antibody therapies currently approved in Korea, including Pfizer's Elrexfio (elranatamab) and Janssen's Tecvayli (teclistamab) and Talvey (talquetamab), remain non-reimbursed. Suk Jin Kim, Professor of Hematology-Oncology at Samsung Medical Center and the President of the Society of Hematology said, “Although treatment outcomes have improved significantly with the active development of new drugs, the limited access to high-priced anticancer drugs have been preventing patients from receiving optimal treatment as needed.” Kim added, “Policy changes are needed, including flexibility in Korea’s reimbursement standards, to ensure that patients in Korea have access to treatment that meets global standards. We must urgently expand patient access to treatment through early adoption of innovative therapies to close Korea’s survival gap compared with other countries.”
- Company
- Ziihera receives orphan drug designation in Korea
- by Eo, Yun-Ho Jan 20, 2025 05:54am
- The first HER2 bispecific antibody drug Ziihera has received an orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced so through the first orphan drug designation in the new year. Its specific indication is for the treatment of adult patients with previously treated unresectable locally advanced or metastatic HER2-positive (IHC3+) biliary tract cancer. Ziihera (zanidatamab), a bispecific antibody that targets the HER2 gene, received accelerated approval from the U.S. FDA in November last year after demonstrating efficacy in patients with biliary tract cancer. This is the first time that a bispecific antibody targeting the HER2 gene has been used as a second-line treatment option for patients with biliary tract cancer who are identified as HER2-positive (IHC 3+). Biliary tract cancer is a fatal disease with a poor prognosis and a low five-year survival rate of less than 5% for metastatic disease. The company demonstrated Ziihera’s efficacy through the single-arm phase IIB HERIZON-BTC-01 study. In the trial, the drug met the primary endpoint of confirmed objective response rate (cORR) by independent central review (ICR). The objective response rate was 52%, and the median duration of response (DOR) was 14.9 months. The safety profile of Ziihera was demonstrated in the HERIZON-BTC-01 trial in 80 patients. During the study, 53% of patients treated with Ziihera experienced an adverse event. The most common adverse events were diarrhea, infusion-related reactions, abdominal pain, and fatigue. Serious adverse events occurring in 2% or more of patients were biliary obstruction, biliary infection, sepsis, pneumonia, diarrhea, gastric obstruction, and fatigue. The trial results were presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting and published in The Lancet Oncology. Long-term follow-up data showing improvement in the duration of response were reported at the 2024 ASCO Annual Meeting. Meanwhile, BeiGene has the domestic rights to Ziihera. The drug is currently being studied in multiple cancers, including Phase III trials in gastroesophageal adenocarcinoma (GEA) and metastatic breast cancer (mBC). It is also currently being studied in the Phase III HERIZON-BTC-302 trial, which compares the combination of Ziihera and standard of care (Soc) to Soc alone in patients with HER2-positive biliary tract cancer.
- Company
- [Reporter' View] Results from J.P. Morgan Conference
- by Lee, Seok-Jun Jan 20, 2025 05:53am
- The Annual J.P. Morgan Healthcare Conference (hereafter J.P. Morgan Conference) has ended. It is the largest funding event in the pharmaceutical and biotech industry and is held in January every year. Many Korean companies have also participated in the event, to the extent that many key R&D people in the Korean pharmaceutical and biotech industry were said to be not present in Korea. According to the J.P. Morgan Conference report, almost 30,000 one-on-one business meetings requested at this year's event were held, where 12,000 agreements have been met. The total market capitalization of 531 companies at the official presentation sessions is US$ 9.6 trillion. Consequently, the J.P. Morgan Conference presents an opportunity for Korean pharmaceutical and biotech companies. According to the J.P. Morgan Conference report, almost 30,000 one-on-one business meetings requested at this year's event were held, where 12,000 agreements have been met. The total market capitalization of 531 companies at the official presentation sessions is US$ 9.6 trillion. Consequently, the J.P. Morgan Conference presents an opportunity for Korean pharmaceutical and biotech companies. Companies referred to as conglomerates in the pharmaceutical industry have unveiled their specific accomplishments. For instance, Yuhan presented its latest data on the new lung cancer drug, Leclaza, through its partner, Johnson & Johnson (J&J). Joaguin Duato, CEO of J&J, said, "A combination therapy of Leclaza and Rybrevant demonstrated results of extending three-year life-expectancy of lung cancer patients over a year. It is a difference that may bring changes to the treatment paradigm." UK-based AstraZeneca's Tagrisso (osimertinib), the standard therapy used in patients with EGFR-mutated non-small cell lung cancer (NSCLC) has the median overall survival (mOS) of approximately 3 years. The combination therapy of Leclaza and Rybrevant is expected to have a mOS of over 4 years. J&J projected the yearly sales goal for Leclaza+Rybrevant to be over KRW 7 trillion. Samsung Biologics announced that it will begin the antibody-drug conjugate (ADC) service in the first quarter of this year. The company aimed to provide top-level Contract Development and Manufacturing Organization (CDMO) services in the ADC field. Moreover, the company announced the building of Plant 6 facility this year. Other companies, including Celltrion, Lotte Biologics, Hugel, and SK Biopharmaceuticals, presented their R&D vision. These companies have shared their success at the J.P. Morgan conference. In contrast, some companies showed a different stance before and after the conference. It is difficult to estimate, but based on yearly trends, more than half of the companies that promoted before the conference did not issue additional press reports afterwards. Despite using promotional keywords, such as promoting technology transports, disclosing growth strategies, conducting big pharma meetings, and establishing facilities, these companies failed to release feedback after attending the J.P. Morgan Conference. The J.P. Morgan Conference has ended. Companies must release their accomplishments following the conference if they genuinely wish to be acknowledged for their company values. Rather than issuing promotional press releases beforehand, they should share objective results afterward. If one issues a promotional subject, providing feedback is essential. Additionally, companies must announce their accomplishments. Disclosable materials are limitless, depending on how companies view them. This week is the best time for companies to showcase their successes at the J.P. Morgan Conference.
- Company
- Expanded reimb for 'Zejula'
- by Son, Hyung Min Jan 17, 2025 05:53am
- Dr. Jae-Weon Kim of Seoul National University Hospital Following the reimbursement expansion of Zejula for ovarian cancer, patient access to treatment has been improved. Experts suggest that Zejula will be more widely used in clinical practices based on demonstrated benefits in efficacy and safety in long-term treatment studies. On January 16, Takeda Pharmaceutical Korea held a press conference commemorating the reimbursement expansion of Zejula monotherapy as a first-line maintenance therapy of HRd-positive ovarian cancer. As of October 2024, the national health insurance reimbursement criteria for Zejula have been expanded to include treatment of homologous recombination deficiency (HRD)-positive ovarian cancer. Previously, Zejula has been covered by reimbursement only as a maintenance therapy for the first-line treatment of patients with ovarian cancer associated with BRCA who respond to platinum-based therapy. Due to the reimbursement expansion, Zejula became the only PARP (Poly ADP-ribose Polymerase)-inhibitor covered by reimbursement for the first-line maintenance therapy used in patients with HRd-positive ovarian cancer. HRd refers to homologous recombination deficiency, a DNA damage repair mechanism. When HRd is positive, cancer cells cannot efficiently repair DNA damage. It is particularly associated with BRCA1/2 gene mutations frequently observed in breast and ovarian cancers. Experts suggest that the clinical prevalence of HRd expression in ovarian cancer is over 50%. Based on a long-term follow-up study of 6.2 years, Zejula demonstrated significant benefits for patients with HRd-positive ovarian cancer. In the clinical study, the HRd patient group treated with Zejula recorded a progression-free survival of 24.5 months, which was markedly different from the 11.2 months of the placebo group. At the five-year mark of treatment, the number of patients who survived without disease progression in the Zejula group was twice as high as that in the placebo group. In the HRd patient group, a statistically significant difference in progression-free survival was observed between those treated with Zejula and those given a placebo up to five years of treatment. "Zejula has demonstrated long-term PFS benefits through the PRIMA trial, its approval study, and follow-up studies. In terms of safety, adverse events were consistent with previous clinical results, confirming its safety even with long-term use," Dr. Jae-Weon Kim of Seoul National University Hospital's Department of Obstetrics and Gynecology stated. In clinical practices, prescriptions for Zejula have been increasing since its reimbursement expansion. Ovarian cancer patients take two 100 mg tablets once daily, while Zejula is the only ovarian cancer treatment available with a once-daily dosing regimen. "Since the reimbursement expansion notice, no severe adverse events have been reported among HRd-positive patients continuing first-line maintenance therapy with Zejula monotherapy. Zejula, as an oral medication administered once daily, significantly enhances patient convenience," Dr. Jung-Yun Lee of Severance Hospital's Department of Obstetrics and Gynecology emphasized. Dr. Lee added, "HRd is a biomarker commonly observed during first-line maintenance therapy. With the reimbursement expansion of Zejula, there has been an increase in cases involving HRd testing, leading to higher diagnosis rates. Through diagnostic testing, many patients are expected to benefit from Zejula." Dr. Jung-Yun Lee of Severance Hospital
- Company
- Ultomiris is reimbursed for aHUS in Korea
- by Son, Hyung Min Jan 16, 2025 06:14am
- Dr. Jinseok Kim, professor of hematology at Severance Hospital. Ultomiris has been reimbursed in Korea for atypical hemolytic uremic syndrome (aHUS) since January. While experts have welcomed the news of its reimbursement, they have also raised the need for systematic improvements to the stringent conditions for reimbursement, including the preliminary review requirement. On the 10th, AstraZeneca Korea held a press conference at JW Marriott Dongdaemun Square Seoul to celebrate the reimbursement coverage of Ultomiris for aHUS in Korea. Starting this month, Ultomiris will be reimbursed by health insurance for patients with aHUS with thrombotic microangiopathy (TMA) and kidney damage. This is expected to improve access to treatment for patients with aHUS whose symptoms can worsen rapidly and lead to end-stage renal disease (ESRD). Ultomiris is a next-generation C5 complement inhibitor with a half-life approximately four times longer than Soliris. While Soliris requires dosing every 2 weeks, Ultomiris has an extended dosing interval of 8 weeks, improving treatment convenience. When complement C5 is activated on the surface of bacteria, it produces a membrane-attacking complex that causes holes in the cell membrane. If the normal immune system process of complement activation continues, vascular endothelial cells are disrupted, causing related diseases. Ultomiris has a mechanism of action that inhibits this process. aHUS is an acute rare disease in which the immune system's complement is overactivated due to a genetic defect and causes thrombotic microangiopathy, which can lead to severe damage to multiple organs, especially the kidneys. aHUS refers to hemolytic uremic syndrome not associated with E. coli. The efficacy and safety of Ultomiris were confirmed in Phase III Study 311 in adult patients who were not previously treated with complement inhibitors. At 26 weeks of treatment, Ultomiris demonstrated improvement in TMA-related markers, including platelet and LDH levels, in 53.6% of patients. The treatment also demonstrated sustained terminal complement inhibition by maintaining serum-free C5 concentrations of < 0.5 μg/ml. In the Phase III Study 312 in pediatric patients, Ultomiris demonstrated complete resolution of TMA in 94.4% of patients at 50 weeks of treatment. “In a study of pediatric aHUS patients who switched from Soliris to Ultomiris, the renal and hematologic parameters remained stable over one year, confirming the efficacy of the switch,” said Dr. Jinseok Kim, professor of hematology at Severance Hospital. “As clinicians, we are excited to be able to offer hope to our patients with the introduction of this new treatment option.” Ultomiris and Soliris are currently available for aHUS in Korea, but both agents are only available to patients who have been approved through the prior review process. Clinicians have called for improved reimbursement, including moving to a post-review process, as patients' conditions can worsen if they are not properly dosed. The coverage of aHUS was implemented in 2018 with the introduction of Soliris. However, according to an analysis of the results of the preliminary review system from July 2018 to October last year, 56 out of 321 cases were approved, showing an average approval rate of 18%. Kim added, “Although Ultomiris has been reimbursed, like Soliris, it is subject to the same restrictions as Soliris, which requires preliminary review. We hope that systemic improvements are also made so that aHUS patients can receive treatment in a timely manner.”
- Company
- Last year's pharma export sales recorded KRW 11T
- by Kim, Jin-Gu Jan 16, 2025 06:14am
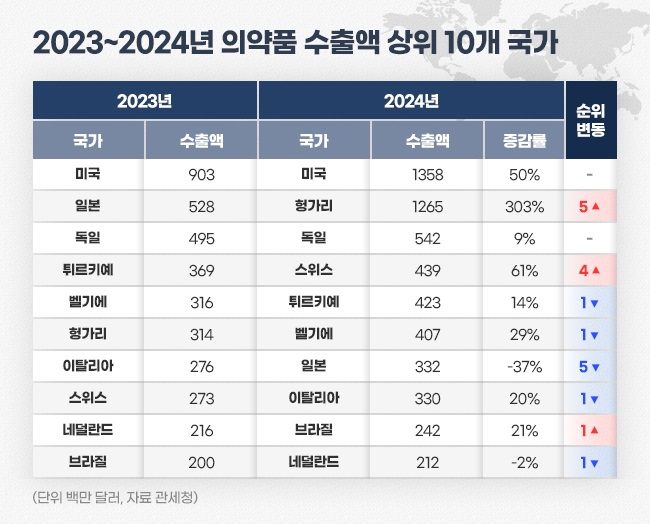
- Last year's export sales of Korea-made pharmaceuticals amounted to US$ 7.5 billion (approximately KRW 11 trillion), up 29% from the previous year. Analysis indicates that a significant increase in biopharmaceutical exports of Samsung Biologics and Celltrion has contributed to the growth. The largest exported country was the United States, with exports totaling US$ 1.3 billion (approximately KRW 1.98 trillion), up 50% from the previous year. Export sales to Hungary increased more than fourfold over the year, reaching US$ 1.2 billion (approximately KRW 1.85 trillion), making it the second largest after the United States. Pharmaceutical export sales reached US$ 7.54 billion last year…second largest in the history According to the Korea Customs Service on January 16, last year's Korean pharmaceutical export sales amounted to US$ 7.53 billion. After the announcement of the endemic, export sales expanded to the largest size. The export sales of domestically produced pharmaceuticals significantly increased during the COVID-19 pandemic. In 2019, the export value was US$ 3.69 billion, which soared by 81% over the year to reach US$ 6.68 billion in 2020 as the pandemic intensified. By 2021, this figure had further increased by 22%, reaching US$ 8.12 billion, setting an all-time high, primarily driven by the export sales of domestically developed COVID-19 vaccines. After that, the sales decreased for two consecutive years until 2023. In 2022, it decreased to US$ 6.27 billion, down 23% compared to the previous year. In 2023, it further shrank to US$ 5.85 billion. Last year, export sales rebounded successfully. Compared to 2023, export sales rose by 29%. It is the second largest after 2022. Yearly pharmaceutical export sales (unit: US$ 1 million, source: Korea Customs Service) The pharmaceutical industry considers that the export growth resulted from a significant increase in biopharmaceutical exports to the United States and Europe, primarily driven by Samsung Biologics and Celltrion. As of Q3 last year, Samsung Biologics' cumulative export sales reached KRW 3.29 trillion, up 26% compared to KRW 2.62 trillion in the Year-over-Year (YoY). This represents an annual export growth of over KRW 600 billion. Considering its order backlog, Samsung Biologics is projected to achieve record-breaking yearly export performance. Since 2015, Samsung Biologics has secured US$ 14.23 billion in orders as of Q3 last year. Among this, US$ 7.49 billion of products have been delivered, leaving an order backlog of US$ 6.73 billion. If the clients successfully develop their products, the estimated value of the order backlog could rise to US$ 12.31 billion. Celltrion's cumulative export sales for Q3 also increased by 30%, from KRW 623.5 billion in 2023 to KRW 810 billion in 2024. Additionally, SK Biopharm, GC Biopharma, Yuhan, Hugel, Dongwha, Daewon, and Boryung also had significant increases in export sales, contributing to the overall growth of pharmaceutical exports. Pharmaceutical import sales reached US$ 8.98 billion, marking a 5% decrease compared to the previous year. The pharmaceutical trade deficit narrowed with a significant increase in exports and a decline in imports. The trade deficit improved from US$ 3.58 billion in 2023 to US$ 1.45 billion last year. Exports to the US represent largest amount over three consecutive years…Hungary, a four-fold increase over the year Korea has exported the most to the United States the most. Last year's pharmaceutical exports to the United States reached US$ 1.35 billion. The United States accounts for 18% of the total pharmaceutical export sales. Export sales to the United States quickly rose over the past two years. It increased 7% from KRW 843.94 million in 2022 to KRW 933.0 million in 2023. Last year, it increased over 50%. In 2022, the United States became the largest export destination, replacing Germany. Since then, it has maintained the top rank for three consecutive years. Hungary ranked second in export sales after the United States. Last year's export sales to Hungary reached US$ 1.26 billion, surging more than four-fold compared to US$ 316.27 million in 2023. The top countries by pharmaceutical export sales 2023-2024 (unit: US$ 1 million, source: Korea Customs Service) One of the reasons for the significant increase in exports to Hungary is Celltrion's expanded biosimilar export to Europe. Hungary is Celltrion's export hub for the European market. The company announced its plans to establish a direct sales system using its Hungarian subsidiary as the gateway for European exports. As part of this strategy, shipments of Celltrion's biosimilars for the European market have been concentrated through its Hungarian subsidiary. Celltrion's biosimilars, primarily Remsima SC, performed strongly in Europe last year. The company's cumulative European export sales for Q3 of last year reached KRW 444.9 billion, up 57% compared to KRW 283.5 billion during the same period the previous year. This growth is attributed to the expanded export coverage of Remsima SC to additional countries. Followed in ranking were Germany, Switzerland, Türkiye, Belgium, Japan, Italy, Brazil, and the Netherlands. Export sales increased YoY for all countries except Japan and the Netherlands. Exports to Germany amounted to US$ 542.14 million, up 9% from US$ 495.40 million in 2023. Exports to Switzerland recorded US$ 438.78 million, up by 61%. Exports to Türkiye rose 14% to US$ 422.80 million, while Belgium saw a 29% increase, recording US$ 406.59 million. Exports to Japan declined by 37% from US$ 528.47 million in 2023 to US$ 331.77 million. In 2023, exports to Japan ranked second after the United States. However, it went down to No.7 for last year.
- Company
- Elahere receives orphan drug designation in Korea
- by Eo, Yun-Ho Jan 16, 2025 06:13am
- The new ovarian cancer drug Elahere has received an orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced the designation through the first orphan drug designation announcement of the new year. Specifically, the drug is indicated as monotherapy in adult patients with folate receptor-alpha (FRα) positive ovarian cancer, fallopian tube cancer, or primary peritoneal cancer who have received one to three prior systemic therapies and have not responded to or are no longer responding to treatment with platinum-based chemotherapy. AbbVie’s Elahere(mirvetuximab soravtansine) first received accelerated approval in the U.S. in November 2022 and then full approval in March of this year based on the MIRASOL clinical data, and was also recently approved in Europe. Ovarian cancer patients initially receive platinum-based anticancer drugs as treatment. However, many patients develop resistance to platinum-based anticancer drugs, which makes it difficult for patients to achieve anti-cancer effects with just platinum-based anticancer drugs. Antibody-drug conjugates (ADCs), which combine an antibody with a platinum-based drug (payload), have been developed in recent years to overcome this issue. Elahere, is a first-in-class ADC drug that combines a folate receptor alpha antibody and the tubulin inhibitor ‘maytansinoid payload DM4’ with a linker. The efficacy of the drug was demonstrated through the Phase III MIRASOL study. In the trial, Elahere improved the primary endpoint of progression-free survival (PFS), with a 35% reduction in the risk of disease progression or death compared to chemotherapy. Elahere also improved the secondary endpoint of overall survival (OS) compared to chemotherapy, with a 33% reduction in the risk of death in the Elahere arm. The objective response rate (ORR) was 42% in the Elahere arm and 16% in the chemotherapy arm. Among those who responded, 5% showed complete responses and 37% showed partial responses. AbbVie added Elahere to its oncology portfolio when it acquired the U.S. biopharmaceutical company ImmunoGen in 2023 for approximately USD 10.1 billion (KRW 14.7561 trillion).
- Company
- Companies develop new drugs for Fabry disease
- by Son, Hyung Min Jan 16, 2025 06:13am
- Domestic and foreign pharmaceutical companies are speeding up the development of new drugs for the rare Fabry disease. They aim to develop treatments that improve not only efficacy and safety but also administration methods compared to existing treatments. According to industry sources on the 15th, the Ministry of Food and Drug Safety recently approved the Phase I/II clinical trial plan (IND) for LA-GLA, a new drug candidate for Fabry disease being jointly developed by GC Biopharma and Hanmi Pharmaceutical. The two companies entered multinational clinical trials in August last year after receiving approval in the U.S. and Korea. Fabry disease is a rare disease that is inherited as a sex chromosome and is a type of Lysosomal Storage Disease (LSD). It is caused by a deficiency of the enzyme ‘alpha-galactosidase A,’ which breaks down glycolipids in lysosomes, an organelle in cells that remove unnecessary substances. The continuous accumulation of unprocessed glycolipids in the body leads to cytotoxic and inflammatory reactions, and progressive damage to various organs, eventually leading to death. LSD is caused by a genetic deficiency of certain enzymes, resulting in metabolic abnormalities. Lysosomes, which are organelles within cells, contain enzymes that break down substances that the body no longer needs. When these enzymes are impaired or not produced, substances that should be broken down gradually accumulate in the cell. Fabry disease, mucopolysaccharidosis, Pompe disease, and Gaucher disease, are typical examples of LSD. In 2020, Hanmi Pharmaceutical and GC Pharma signed an agreement to jointly develop next-generation innovative drugs for the treatment of LSD and are conducting joint research with the goal of developing global innovative drugs in the field of rare diseases. LA-GLA, a new drug candidate for Fabry disease being developed by the two companies, is a next-generation long-acting enzyme replacement therapy that improves the limitations of existing treatments. GC Biopharma and Hanmi Pharmaceutical plan to develop LA-GLA into a subcutaneous injection formulation that can be administered once a month. Most Fabry disease patients are treated with enzyme replacement therapy (ERT), an intravenous injection of enzymes developed by gene recombination technology. Sanofi Currently, Sanofi's Fabrazyme, Takeda's Replagal, Handok's newly introduced drug Galafold, and ISU Abxis’ Fabrazyme biosimilar Fabagal are being currently used in Korea. All but Galafold are intravenous ERT formulations. Galafold is an oral formulation that is more convenient to administer, but it is not available until after a year of ERT-based therapy. ERT-based therapies are known to have limitations, including the inconvenience of long intravenous infusions every 2 weeks in the hospital and the lack of efficacy in controlling advanced kidney disease. In preclinical studies, LA-GLA has been shown to improve kidney function and fibrosis compared to existing therapies, and in an animal model with Fabry disease, LA-GLA significantly improved peripheral sensory function and histologic lesions of the nerve cells that control it and was effective in improving the increase in blood vessel wall thickness caused by glycolipid accumulation. LA-GLA was designated as an orphan drug (ODD) by the U.S. Food and Drug Administration (FDA) in May based on its efficacy in improving kidney function, vascular disease, and peripheral nerve disorders compared to existing therapies in the clinical stage. Gene therapy also in development The global pharma and biotech industry also has the potential for new drugs for Fabry disease through single-dose gene therapy. Last month, the FDA granted orphan drug designation to Exegenesis Bio’s Fabry disease drug candidate 'EXG110'. EXG110 is a single-dose gene therapy candidate designed to clone the GLA gene and deliver it to liver and heart cells. The drug candidate utilizes an adeno-associated virus (AAV) to deliver the therapeutic gene. AAV is a virus that does not cause disease in humans and is characterized by its ensured safety. Exogenesis is currently conducting clinical trials in Fabry disease patients in China. The first patient has now been dosed with EXG110. The company plans to seek approval in the U.S. for a multinational trial. In September of last year, Unicure's Fabry disease drug candidate AMT-191 was granted orphan drug designation in the United States. AMT-191 is a gene therapy designed to target the liver to produce the GLA protein. Unicure is conducting a Phase I/II clinical trial of AMT-191 in the United States. Unicure plans to administer AMT-191 to 6 patients with Fabry disease to measure the expression of the lysosomal enzyme aGLA-A and evaluate the safety, tolerability, and efficacy of the candidate.
- Company
- JW Pharma's hemophilia assay for patients using 'Hemlibra'
- by Kim, Jin-Gu Jan 16, 2025 06:13am
- Product photo of Hemlibra. JW Pharmaceutical announced on January 15 that its chromogenic assay designed to assess the severity of 'hemophilia' in patients using 'Hemlibra' has been commercialized in South Korea. The Ministry of Health and Welfare (MOHW) has newly established a non-reimbursable CSA testing criterion, effective January 1. CSA test is used to measure the activity of clotting factors in patients with hemophilia to diagnose the severity of the disease and to monitor the effectiveness of the treatment. It is particularly effective in accurately measuring factor VIII, which is deficient in patients with Hemophilia A. It is suitable for patients who use non-coagulant agents like Hemlibra. The primary test used in South Korea is the One-Stage Clotting Assay (hereafter, OSA test). However, the OSA test is limited in accurately diagnosing patients with mild symptoms and confirming the severity of hemophilia in patients treated with Hemlibra. For this reason, the World Federation of Hemophilia (WFH) recommends a combination of OSA and CSA. CSA testing is recommended for testing the severity of hemophilia in patients using Hemlibra and hemostasis testing after factor VIII combination therapy. "The commercialization of the CSA test allows for assessing hemophilia severity in patients treated with Hemlibra," said Dr. Park Young-sil, professor of pediatrics at Kyung Hee University Hospital at Gangdong. "It will also improve the diagnostic environment for patients with mild symptoms and female hemophilia patients (carriers) who could not be accurately diagnosed using the existing OSA test." "The commercialization of the CSA test will significantly advance hemophilia diagnosis and treatment," said a representative from JW Pharmaceutical. "We will strive to improve patient access to treatments and the quality of life for patients with hemophilia A."
- Company
- China-made new drugs actively enter the global market
- by Son, Hyung Min Jan 16, 2025 06:13am
- Many Chinese pharmaceutical and biotech companies are demonstrating results from their new anti-cancer drug development in the global market. Companies like Jiangsu Hengrui Pharmaceuticals and BeiGene have obtained approvals for their immunotherapy and targeted anti-cancer agents from the regulatory authorities globally, including South Korea, the United States, and Europe. The gap between Chinese and Korean pharmaceutical and biotech companies has increased as Chinese companies succeeded in commercialization and Phase 3 clinical trial entries. It has been reported that most new anti-cancer drugs from Korean pharmaceutical and biotech companies remain in the preclinical stages or phase 1 clinical trials. Two immunotherapy drugs have been commercialized in the U.S….bispecific antibodies have entered phase 3 trials According to industry sources on January 14, the US Food and Drug Administration (FDA) recently approved 'Tevimbra,' an immunotherapy developed by a Chinese pharmaceutical company, BeiGene, as a first-line treatment for HER2-positive gastric cancer. Following the approval, Tevimbra became the third immunotherapy following MSD's Keytruda and BMS·Ono Pharmaceutical's Opdivo to be used as a first-line treatment for HER2-positive gastric cancer. In November 2023, Tevimbra received domestic approval as a monotherapy for the treatment of adult patients with unresectable, recurrent, locally advanced, or metastatic esophageal squamous cell carcinoma who are unable to continue platinum-based chemotherapy or have experienced recurrence or progression following such treatment. BeiGene has started over 17 clinical trials for potential indications for Tevimbra. The company has already confirmed positive results from 11 phase 3 trials and 4 phase 2 trials. To date, over 900,000 patients have been treated with Tevimbra. Tevimbra obtained approvals from over 40 countries worldwide. Chinese pharmaceutical companies had previously failed to obtain approvals from global regulatory authorities when only enrolling Chinese patients. Now, these companies are overcoming the approval hurdles by enrolling patient groups of various ethnicities and genders. In November 2023, 'Loqtorzi,' anti-PD-1 immunotherapy from China's Junshi Biosciences, was approved in the United States. Loqtorzi has been approved as the first-line treatment in combination with platinum-based chemotherapy for the treatment of metastatic or locally advanced nasopharyngeal carcinoma (NPC). Junshi Biosciences entered into an out-licensing deal with the US-based Coherus BioSciences for Loqtorzi with an upfront payment of US$ 150 million, totaling US$ 1.1 billion (approximately KRW 1.6 trillion). Jiangsu Hengrui Pharmaceuticals aims to receive approval for its camrelizumab in combination with the HLB group's targeted anti-cancer, Rivoceranib. In May 2024, Jiangsu Hengrui Pharmaceuticals and HLB received a Complete Response Letter (CPL) from the FDA but received a decision of 'not required to take additional actions' during the site monitoring. The combination therapy containing Rivoceranib and Jiangsu Hengrui Pharmaceuticals' immunotherapy, camrelizumab, demonstrated the longest survival extension benefit as the first-line treatment for liver cancer. China's Innovant is knocking on the door of the FDA in collaboration with the global pharmaceutical company Eli Lily. Following obtaining approval for the immunotherapy sintilimab in China, Innovant signed an out-licensing agreement with Eli Lily in 2015 worth US$ 1 billion (approximately KRW 1.3375 trillion). Both companies applied for approval of sintilimab for the treatment of non-small cell lung cancer (NSCLC) but failed to obtain the FDA approval. Lily and Innovant plan to conduct multi-regional clinical trials comparing sintilimab+chemotherapy to the existing standard therapy as requested by the FDA. Chinese pharmaceutical companies are showing results in bispecific antibodies. Last year, the PD-1/VEGF bispecific antibody ivonescimab demonstrated superior effects than Keytruda. Based on a Phase 3 clinical trial involving 398 patients with PD-L1-positive NSCLC, ivonescimab reduced the tumor progression risk by 49% than Keytruda. Ivonescimab is a bispecific antibody developed by China's Akeso, and Akeso signed a partnership agreement with the US-based Summit Therapeutics. Summit Therapeutics has the right to develop ivonescimab in the U.S., Canada, Europe, Japan, and Latin America. Akeso is developing AK112, a new drug candidate, in China and Australia. Chinese biotech company SystImmune is developing 'BL-B01D1,' targeting EGFR and HER3, which are commonly found in solid cancers. The company has confirmed the efficacy and safety in clinical trials and out-licensed the candidate to BMS for US$ 8.4 billion (KRW 11.7 billion). Korean companies' candidates are in the early phases of clinical trials…increasing gap between China Chinese companies have successfully commercialized their immunotherapies and bispecific antibodies. However, Korean pharmaceutical and biotech industry candidate products are still in the early phases of clinical trials. TiumBio is conducting a Phase 2ab trial of its immunotherapy candidate TU2218. TU2218 blocks pathways of transforming growth factor beta (TGF-ß) and vascular endothelial growth factor (VEGF), which are known to hinder cancer immunotherapy activation. In a Phase 1a trial, TU2218 treatment reduced the blood concentration of major biomarkers of connective tissue growth factor (CTGF), whose expression is known to be regulated by TGF-ß. After seven days, CTGF blood concentration decreased by 27% on average compared to baseline levels. Currently, in addition to the TU2218 monotherapy, TiumBio is also conducting clinical trials for a combination therapy that includes Keytruda. GI Innovation recently modified its Phase ½ clinical trials in the United States studying immunotherapy candidate 'GI-102' to confirm its combination with Enhertu. GI-102, which acts on CD80 and interleukin (IL)-2. IL-2 is involved in immune cell proliferation and activation, and CD80 blocks CTLA-4, a receptor preventing immune cells from attacking cancer cells. GI-102 is being developed as intravenous (IV) and subcutaneous (SC) formulations. GI-102 demonstrated potential in a monotherapy clinical trial. According to Phase 1/2a clinical trial data presented by the company, Skin melanoma patients treated with GI-102 had an objective response rate (ORR) of 43%. Under GI-102 treatment, lymphocyte proliferation was found, and there has been no significant drug toxicity regarding the safety profile. ImmuneOnsia confirmed the drug safety profile of its immunotherapy candidate, IMC-002, is a dose-escalation Phase 1a trial. IMC-002 works by blocking the signal between CD47 of cancer cells and macrophages. The company monitored the effect of IMC-002 in 12 patients, and there was no drug toxicity in each dosage. Six out of 12 patients had steady disease (SD). The company plans to determine the recommended dosage for the phase 2 trial based on the results from the phase 1 trials. ABL Bio has started a phase 1 trial to evaluate the efficacy and safety of its immunotherapy candidate ABL103 in patients with advanced·metastatic solid cancer. The company aims to evaluate the safety and tolerability of ABL103 monotherapy and determine the recommended dosage and maximum tolerated dose for a subsequent phase 2 clinical trial. The phase 1 trial will be conducted in both South Korea and the United States. In preclinical studies, ABL103 demonstrated to induce 4-1BB activation in tumor microenvironments expressing B7-H4. Additionally, it showed complete eradication of cancer cells and the suppression of recurrence in homologous cancer cells. Pharmaceutical personnel said, "New drug candidate discovery capacity of Korean companies is at the global level. However, they have difficulty entering a later phase of clinical trials. To secure global approval, these companies need to conduct multi-national clinical trials and clinical trials across numerous sites. Therefore, raising the funds can be difficult." "Companies must demonstrate the benefits of new drug candidates and how to address their weaknesses in clinical trials. They should identify unmet needs in clinical practices before conducting trials and set goals for the development of new drugs based on intended purpose," he added.