- LOGIN
- MemberShip
- 2025-12-22 08:23:45
- Company
- ‘Employees work long-term at Daiichi Sankyo for a reason'
- by Eo, Yun-Ho Feb 13, 2025 05:58am
- To survive and develop, we need to change and adapt to change. This is easy to say, but never easy to do. Daiichi Sankyo is a master of adaptation. The company has established itself in the domestic market by supplying drugs for chronic diseases, mainly cardiovascular diseases. Starting with hypertension drugs such as Olmetec, Sevikar, and Sevikar HCT, the company has launched the antiplatelet drug Effient and anticoagulant Lixiana and has achieved continuous growth by launching new leading products in crisis, such as patent expiry. Maybe it's the products, but it's also the strategy. The story of how Daiichi Sankyo stopped promoting its antibiotic Kravit independently when Olmetec’s patent expired and focused on its circulatory system pipeline through co-promotion with a Korean company is still much talked about. Now, Daiichi Sankyo is looking to take a new leap forward with its oncology business. Last year, Enhertu, an antibody-drug conjugate (ADC) that was listed for reimbursement in Korea, began to gain popularity in the anticancer field, supported by its excellent clinical results. With an average annual growth rate of 15% from 2014 to date, Dailypharm sought to learn more about the talents behind the company's growth. Daily Pharm met with Mr. Hyundong Ryu (50), Managing Director of Management Headquarters at Daiichi Sankyo to learn more about the people behind the company’s growth. Hyundong Ryu, Managing Director of Management Headquarters, Daiichi Sankyo Korea-Tell us about the company's organizational structure. What does the Management Headquarters do? Daiichi Sankyo Korea has 4 headquarters. The Primary Business Unit, responsible for chronic diseases and specialty drugs; the Oncology Business Unit, is responsible for anti-cancer drugs; the Development Medical Headquarters, which is responsible for clinical development, licensing, and follow-up; and the Business Management Headquarters, which is responsible for the overall management and operation of the company. I am currently in charge of the entire Business Management Headquarters, which includes human resources, general affairs, finance, distribution, IT, business planning, and external relations. -Daiichi Sankyo is very good at coming up with products that are needed at the time and achieving good results with those products. This cannot be done without the right “people.” Daiichi Sankyo is known for its job longevity, especially in the industry. What is the secret behind this? We have our own management philosophy and values with regard to human resources, on how to develop talent and treat them at Daiichi Sankyo. We don't just run a company for business, for sales, but for people to come together and work together. The mindset of not using people as tools is something that has been passed down as part of our culture. I think the sincerity of the management in valuing talent and caring to not make employees feel like they are being used as a tool is one of the important reasons behind our employees’ job longevity. -Regarding the new Oncology BU, it’s not easy to create a business unit that didn't exist before, get it up and running, and then have it drive the company's future strategy. What did you focus on and what difficulties did you face in creating the Oncology BU? When you create a new business unit (BU), you're bringing in new people, and the success of the business depends on them. We've been through this process twice in the past where new people come in, intertwine with existing employees, and grow. The company has learned from these experiences how to integrate and resolve cultural conflicts that can arise when bringing in new businesses and bringing in a lot of new talent. We believe that it is best to provide as many opportunities for communication as possible to help people understand the value of the organization's values of 'together' and 'togetherness' rather than trying to artificially seal conflicts. #SB-You seem to have a good internal mindset to deal with change. What does Daiichi Sankyo look for in its employees? Daiichi Sankyo Korea has created new business units and recruited new talents, so in Korea, since the 2020s, we have emphasized and valued “Collaborate & Trust” the most. We don't look at a candidate's experience or specifications but rather how they want to contribute to our organization. For example, we try to select candidates who value work and take responsibility for their work, rather than candidates who emphasize the resonance of “I did it” or “I am the best” or those who want to prioritize their career path. - Interest in multinational pharmaceutical companies has been rising these days. There aren't many new hires at each company, but in the overall framework of multinational companies, this seems to be happening to some extent. Does Daiichi Sankyo have any procedures or requirements for hiring new employees? We hire new employees on a case-by-case basis. It's not a regular program, but we have an intern program that has been up and running for more than 10 years. We hire people who have a good work attitude and understand the value of “working together” as marketing representatives (MRs). We don't look for any specific qualifications. Unless we need a pharmacy professional, we look for a 4-year graduate with some language level, etc., and their attitude towards work and contribution to the team and organization's goals is important. -What programs do you offer to foster talent after they join the company? In terms of fostering talent, the company has a systemic roadmap in place for overall talent growth and support. When you first join the company, we spend 2-3 days training you on the company's systems and other things you need to learn. At the one-year mark, we have a week-long training session at a separate location with other new hires and experienced employees. This is called the first-year onboarding training, which focuses on improving your understanding of the company's mission, values, and culture. In the second year, we hold a reminder training (vision training) where employees reflect on the goals and visions they set in the first year and what they want to achieve within the company. In the third year, you go to the headquarters in Japan for a week of training. When it comes to work-level job training, some parts cannot be fully covered in-house, so we offer training support without a specific budget limit for all training needs. Individuals can request individualized competency training, which they discuss with their manager and then receive support from the company. At the manager level, we offer an annual 360-degree, multi-faceted assessment to identify strengths and weaknesses and provide one-on-one coaching on how to overcome them. Key talent, such as potential next-generation leaders, is developed separately by HR. For example, we support MBA and pharmacy-related graduate programs. There is also a program called STDP (Short-Term Development Program) to help employees gain a global perspective. This program is mainly offered at our Japanese headquarters but has recently been expanded to include offices in the ASCA region (Asia and South America). -Is there a system in place that encourages employees to move between departments? Many programs in the pharmaceutical industry allow people to experience different roles. We have a very active job posting program. When we have a vacancy, we encourage job posting, preferably among existing employees. It is difficult to verify the potential and capabilities of people who have specialized experience from outside the company. On the other hand, we have expectations that people with sufficient internal potential can perform well if they are given time to develop their careers, and this has proven to be true in many cases. -How are employees and executives evaluated? We perform two main types of evaluations: Performance Evaluation and Competency Evaluation. First, in performance evaluation, the basic framework is performance-based relative evaluation for sales and absolute evaluation for internal employees. However, for both sales and internal employees, we make sure that the attitude and effort in the process of performance are fully reflected, not just the arithmetic results. After setting goals, we conduct feedback interviews about four times a year. Midterm feedback is used to correct the direction and track the process of achieving goals. -I'm also curious about the company’s reward system such as incentives. There are two types of performance pay: individual performance pay and management performance pay. Individual performance pay is based on the achievement of individual or team goals. For the base salary, many multinational companies adopt the merit increase system and set the increase rate according to performance, but Daiichi Sankyo does not differentiate the base salary increase rate by performance. We set the same base salary increase for all employees, reflecting the inflation rate of the previous year, and top it up with a performance bonus based on the performance of the organization. This is because cross-functional collaboration between teams and departments is of utmost importance. -Do you have annual longevity awards in addition to incentives? There are 5-yearly awards for 10, 15, 20, and 25 years of service. No one has reached 30 years of service yet, but there will be rewards when they do. For 10 years of service, you get a 10-day sabbatical. It's a valuable reward for contributing to the growth and development of the company over a long period of time, and in fact, long-term service is not uncommon in our company compared to other foreign companies. - The slogan “Cooperation” seems to be at the core of the company's foundation. That's right. At Daiichi Sankyo, individual achievement is important, but the performance of the underlying organization is also important, and this permeates the entire company as a corporate culture. The company takes its time and encourages individuals to develop their capabilities over time. The company focuses on “steadiness” rather than chasing short-term goals. We are creating a corporate culture where each person is not engrossed in their own role but rather has a company-wide perspective, that naturally leads to cooperation and trust.
- Company
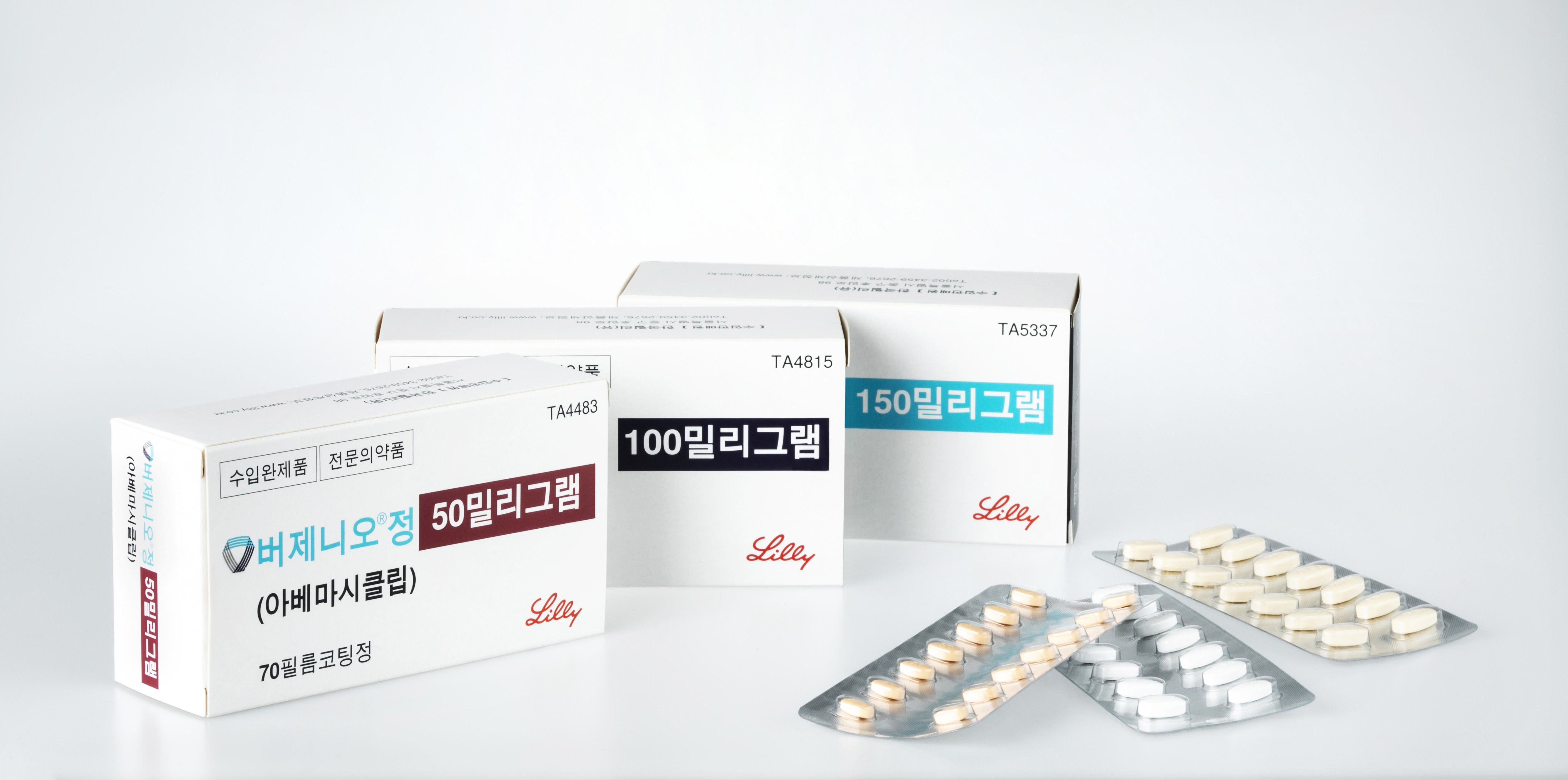
- Will 'Verzenio' be reimb for early breast cancer in 2025?
- by Eo, Yun-Ho Feb 12, 2025 06:13am
- Product photo of Verzenio The industry eyes whether 'Verzenio,' with expanding reimbursement challenges, will obtain results this year. According to sources, Lilly Korea's CDK4/6 inhibitor Verzenio (abemaciclib) is expected to be considered for the upcoming Cancer Disease Review Committee (CDRC) of the Health Insurance Review and Assessment Service (HIRA) for its indication for early breast cancer. Verzenio faced challenges in the first attempt at the CDRC review for its indication to treat early breast cancer. Despite submitting the application and waiting for six months, Verzenio was presented to the committee in May 2023, but the result was 'reimbursement standards non-established.' After five months, Verzenio re-submitted its reimbursement application to the HIRA in October. Then, the drug was considered for the CDRC, but the result was the same as before. Patients have high hopes for reimbursement approval of Verzenio for early breast cancer. In fact, the public petition yielded over 50,000 votes. The efficacy of the drug was demonstrated again through the five-year outcomes from the monarchE study, which was presented at the 2023 European Society for Medical Oncology (ESMO) Congress. The data used for the follow-up research were based on the four-year data presented at the 2022 San Antonio Breast Cancer Symposium held in December and an article published in The Lancet Oncology. The primary endpoints, which were invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS), showed clinically significant differences between the Verzenio treatment group and the control group (endocrine therapy alone) that was even more pronounced in five-year data compared to the four-year data. In year 5, the primary endpoint invasive disease-free survival (IDFS) demonstrated an approximately 8% difference. Verzenio appears to have a potential carry-over effect through the fifth year, even after completing the two-year treatment period. Besides the endocrine therapy letrozole generic, Verzenio is the only treatment option available in HR+/HER2- type early-stage breast cancer. On November 18, 2022, Verzenio was approved for expanded use in combination with endocrine therapy in the adjuvant treatment of patients with HR+/HER2- high-risk early-stage breast cancer and who have lymph node-positive recurrence. The following are specific indications: ▲Four or more lymph node metastases, ▲1-3 lymph node metastases with a tumor size of 5 cm or larger, ▲Histological grade 3 limited recurrent high-risk patients. Professor Keun Seok Lee, Professor at National Cancer Center's Center for Breast Cancer Korea, said, "The use of Verzenio in combination with endocrine therapy is recommended by the Korean and international chief guidelines as a post-surgical adjuvant therapy for relapsed and high-risk patients, based on significant evidence. Since the drug's clinical usefulness has been confirmed through clinical studies and chief academic reviews, we need improvements to patient treatment access through reimbursement to increase the survivability of patients who qualify as relapsed and high-risk conditions."
- Company
- 'Lorviqua' accepts DREC condition…negotiations to start
- by Eo, Yun-Ho Feb 11, 2025 06:02am
- Product photo to Lorviqua The final review process is about to begin for expanded reimbursement of the non-small cell lunger cancer treatment 'Lorviqua' as a first-line treatment. According to industry sources, Pfizer Korea accepted the condition of 'accepting the drug price below the evaluated amount' suggested by the Drug Reimbursement Evaluation Committee (DREC) of the Health Insurance Review and Assessment Service (HIRA) in January regarding the ALK anticancer drug, Lorviqua (lorlatinib). The company will soon undergo drug price negotiations with the National Health Insurance Service (NHIS). The Ministry of Health and Welfare (MOHW) has recently issued a green light for negotiations, and they will begin the process this month. As the company terminated the Risk Sharing Agreement (RSA) for Lorviqua, switching it to general medicine reimbursement, it is proceeding with the expanded reimbursement for first-line treatment. After the breakdown of the drug price negotiations with the NHIS in June, Pfizer immediately filed for general medicine reimbursement, which passed the last HIRA's DREC for 2024. Even after reapplication, the reimbursement process has been slow. Analysis suggests that the government postponed the decision because Lorviqua was initially contracted as a total expenditure-capped RSA, then switched to a general medicine reimbursement. The industry is to closely watch whether Lorviqua will finish the drug price negotiations without additional hurdles and obtain expanded reimbursement. Meanwhile, Lorviqua is a drug developed to penetrate the blood-brain barrier (BBB). The clinical value of the drug has been assessed as significant based on the long-term follow-up results of the CROWN study, which was recently presented at the ASCO. The study results showed that Lorviqua reduced the disease progression and death risk by 81% compared to crizotinib, and 60% of treated patients were alive without disease progression after five years. The risk of brain metastasis was reduced in 94% of the treated patients, with only 4 out of 114 patients who did not previously had brain metastasis developing it after being treated with Lorviqua.
- Company
- Mitsubishi Tanabe Pharma sold to private equity fund
- by Whang, byung-woo Feb 11, 2025 06:02am
- The sale of Japanese pharmaceutical company Mitsubishi Tanabe Pharma to a global private equity firm is expected to bring changes to its Korean branch as well. Although the company is being sold, it is not being sold by business unit, so its effect on the overall market is expected to be small. Rather, the sales are expected to increase support for the company and add strength to its market expansion activities. According to industry sources on the 11th, global private equity firm Bain Capital signed an agreement with Mitsubishi Chemical, the parent company of Mitsubishi Tanabe Pharma, to acquire Mitsubishi Tanabe Pharma. The transaction is valued at approximately ¥510 billion (KRW 4.9 trillion) and will be in a carve-out transaction from its parent Mitsubishi Chemical Group. Carve-out deals typically involve a company evaluating and selling off a specific business unit to raise capital and improve operational efficiency. This involves creating a structure that allows the business unit to operate independently and then selling it off. The advantage for investors is that a standalone business unit has greater growth potential and offers the opportunity to become more competitive in the market. In fact, this transaction was reportedly decided by parent company Mitsubishi Chemical Group to improve management efficiency and focus on its core business. Mitsubishi Chemical Group decided to exit its pharmaceuticals division as part of a reorganization of its business portfolio due to deteriorating profitability in recent years. As the pharmaceutical division requires long-term investment due to its high research and development (R&D) costs, the group has reportedly decided to prioritize and focus on its core chemicals business. Mitsubishi Tanabe Pharma has garnered attention for its successful sales of ALS drug Radicava in the U.S. market, but the limited exclusivity period in the U.S. until 2029 has raised concerns about the company’s long-term growth. As a result, the company needed new investments and strategies to develop additional new drugs and expand its global reach. However, there are predictions that the acquisition will not drive major change as it is not a spin-off. Instead, the company’s focus will be on market expansion as a standalone company. In this regard, Bain Capital has stated that it will acquire Mitsubishi Tanabe Pharma and strengthen the company's R&D capabilities, and support its global market expansion strategy. “We will actively support Mitsubishi Tanabe Pharma to continue its innovation as an independent company and explore new growth opportunities,” said Bain Capital. However, the sale is expected to have some impact on the Korean market. Mitsubishi Tanabe Pharma Korea has been supplying various treatments in Korea and generated about KRW 70 billion in sales last year. Pic of Uplizna The company took active steps last year to secure new growth engines, such as starting the domestic marketing authorization process for the ALS (Amyotrophic Lateral Sclerosis) drug Radicava (edaravone) and initiating the insurance reimbursement process for the optic neuromyelitis drug Uprisna. However, the sale may result in adjustments to its product supply schedule and new drug launch strategy in Korea. Bain Capital and Mitsubishi Chemical plan to finalize the process in the third quarter. Even if Mitsubishi Tanabe Pharma Korea’s business remains intact, there is a possibility of delays in approval and reimbursement discussions, given the administrative procedures. “All the details that were shared internally, including the sales of the business division, were not much different from the global announcement. So although the company’s name will change, I don't think there will be any major changes,” said an industry official. He added, “However, as Bain Capital has expressed its intention to make aggressive investments in the pharmaceutical division, which has been suffering due to funding difficulties of its parent company, there are some positive expectations as well."
- Company
- OliX licenses out RNA-based MASH drug technology to Lilly
- by Feb 10, 2025 05:50am
- OliX, a company developing RNA-based new drugs, has transferred its metabolic dysfunction-associated steatohepatitis (MASH) and obesity drug candidate to the multinational pharmaceutical company Eli Lilly. On the 7th, OliX announced that it had signed a joint development and technology export agreement with Lilly for its MASH and obesity drug candidate 'OLX75016 (OLX702A)'. The total contract size is USD 630 million (about KRW 911.7 billion). The amount is the sum of the upfront payment and the development and commercialization milestones based on clinical progress. Details such as the proportion of the upfront payment were not disclosed. Under the agreement, OliX will continue with the Phase 1 clinical trial of OLX75016. Lilly will be responsible for other research, development, and commercialization. The agreement includes a provision that, upon signing, OliX will grant an exclusive license to Lilly. Specifically, if OliX develops a treatment that targets the 'MARC1' gene and one or more other genes involved in MASH simultaneously, Lilly will have priority rights to that treatment. The company explained that the total size of the contract could increase or exclusive negotiations could be conducted according to the clinical progress. OLX75016 is a candidate for the treatment of obesity and MASH, which is based on double-stranded small interfering RNAs (siRNA) technology. OLX702A is being developed as a subcutaneous injection formulation for the treatment of obesity that is administered once every 3 months. Results of OLX702A in obese animal model (Source: OliX) OliX is currently conducting a Phase I trial in Australia for OLX75016. It began administering the first patient for the Phase I trial in February last year. In May of the same year, it completed a change in the clinical trial protocol to add patients with nonalcoholic fatty liver disease (NAFLD) to the trial subjects to ensure safety and preliminary efficacy. The company aims to complete the first phase of clinical trials this year. OliX previously confirmed the effects of OLX702A on improving fatty liver and liver fibrosis and weight loss in preclinical studies. OliX also confirmed the weight loss synergistic effect when OLX702A in combination with Lilly's ‘Zepbound’ in a high-fat diet obesity mouse model. Zepbound is a dual agonist that simultaneously activates the glucagon-like peptide-1 (GLP-1) receptor and the Glucose-dependent insulinotropic polypeptide (GIP) receptor.
- Company
- Bayer Korea marks 70th year, preparing for 100-yr milestone
- by Whang, byung-woo Feb 10, 2025 05:50am
- Bayer Korea, celebrating its 70th year in business in the Korean market, is preparing for the next leap toward the 100th year. The company plans to enhance the capacity of treatments already launched in the market and its new growth driver pipeline, aiming for long-term and continuous business growth. Additionally, as the importance of the ESG business has been stressed, the company pursues sustainable business by setting specific directions. Bayer launched in South Korea after the Korean war…Bayer known for Aspirin Bayer Korea, the South Korean subsidiary of the global life sciences company Bayer, entered the Korean market in 1955, starting with its crop protection business. In the 1950s, during the post-Korean War period when food shortages were severe, Bayer Korea collaborated with Dongbu Farm Hannong (previously, Korean Agricultural Association) to enhance agricultural productivity. The company’s primary focus was providing various crop protection products to improve crop yields. In the 1960s, Bayer products began manufacturing in South Korea through a technology partnership with Hanil Pharmaceutical. This period marks the introduction of Bayer products into the Korean market. The representative product at the time was aspirin, a widely used pain reliever with over 120 years of history. Historical records also indicate that Bayer contraceptives were distributed to public health centers in South Korea. Bayer's full-scale business expansion took place in 1972 with the establishment of Bayer Pharmaceuticals Korea. The company acquired 30 locally produced products from Hanil Pharmaceutical, including Bayer Aspirin, extending its business into the healthcare sector. In 1989, Bayer Korea was officially established as a corporation. Entering the 2000s, the company expanded its portfolio through successive mergers and acquisitions, including Aventis CropScience, Roche's OTC division, Schering Korea, MSD Consumer Care, and Monsanto. These acquisitions solidified Bayer Korea's position as a leading global life sciences company with a broader and more advanced product lineup. Bayer As of February 2025, Bayer Korea holds a total of 62 pharmaceutical products, including prescription and over-the-counter (OTC) medications. One of Bayer Korea's key products in recent years is Eylea (aflibercept), a treatment for age-related macular degeneration (AMD). In 2023, Eylea is a blockbuster medication that generated KRW 96.8 billion in sales in South Korea. However, the company faces increasing competition from next-generation therapies and biosimilars that challenge Eylea's market dominance. Expanding sales growth remains one of Bayer Korea's concerns. Based on the company report, Bayer's sales for the past four years amounted to ▲ KRW 332.6 billion in 2020 ▲ KRW 340.1 billion in 2021 ▲KRW 358.0 billion in 2022 ▲ KRW 347.6 billion in 2023. Bayer Korea entered the Korean market in 1955 and its current corporation was established in 1989 Bayer in need of a new growth driver…prepares for a new generation of pharmaceuticals As the pharmaceutical landscape continues to evolve rapidly, Bayer has been transitioning into a more agile organization since last year to adapt to environmental changes quickly. An agile organization breaks down departmental barriers, forming multifunctional teams integrating marketing·sales·operations within the same division. This structure enables greater flexibility and responsiveness to meet the demands of a fast-changing market environment. A Bayer Korea representative said, "Bayer Korea has been focusing on strengthening the foundation for collaboration among employees to build a better future together." One of the most significant changes for Bayer Korea is the appointment of JinA Lee as the company’s first Korean CEO since its entry into the Korean market. Lee's appointment highlights the increasing importance of the Korean market and its strong R&D ecosystem, including early- and late-stage clinical trials and real-world data (RWD) studies. Bayer Korea A notable achievement under Lee's leadership is Kerendia, ranked second in outpatient prescription sales among products that received reimbursement in 2024, making a strong debut in its first year as a reimbursed drug. Additionally, high-dose Eylea (8mg), which extends dosing intervals to 20 weeks, entered the reimbursement list earlier than expected in October last year, helping Bayer maintain its influence in the retinal disease market. Also, Verquvo, a heart failure treatment, is established as part of a new generation of therapies, securing Bayer's future growth. This year, Bayer Korea plans to address unmet medical needs in the Korean market and improve patient access to innovative drugs, such as Nubeqa, which has strong market potential. A Bayer Korea representative stated, "In the healthcare sector, Bayer is committed to delivering innovative products and services through research and development that spans disease diagnosis to prevention and treatment. We are strengthening our expertise in cardiovascular, renal, and oncology treatments while expanding into new therapeutic areas with novel treatment options to ensure continuous growth." Bayer Korea aims for the next leap, focusing on 'sustainable mangagement' Bayer Korea, as it celebrates its 70th anniversary, focuses on 'sustainability' for long-term success. In addition to corporate social responsibility activities, the company is committed to sustainable organizational growth, driven by employee-led campaigns. According to Bayer Korea’s report released in December, the company is actively engaged in activities aligned with its four sustainable development goals: ▲Ending hunger ▲Healthcare ▲Climate change response ▲Gender equality and diversity. For instance, the company pursues hunger relief efforts through a partnership with World Vision, supporting underprivileged children through the "Love Lunchbox" volunteer program. This initiative has been expanded into a year-long challenge, where a team of seven employees participates monthly in preparing meal ingredients, cooking, packaging, and cleaning up. Bayer Korea Additionally, volunteer activities such as an art contest for individuals with developmental disabilities, the Unity Marathon with visually impaired participants, and the Proper Disposal of Expired Medicines Campaign were conducted last year. Lee stated, "Bayer Korea's sustainability report holds the greatest significance not in the scale of its outcomes but in our employees' voluntary and active participation. Through this initiative, we have collectively shared Bayer's vision and goals across the company, systematically organized our achievements, and laid the groundwork for developing more advanced strategies in the future." Lee added, "Bayer Korea is undergoing a transitional phase as we introduce a new generation of pipeline products. We aim to successfully establish these new treatments in the market to improve patients' lives while building a strong foundation to develop further innovative therapies."
- Company
- New TED drug 'Tepezza' under review for marketing approval
- by Eo, Yun-Ho Feb 10, 2025 05:50am
- Product photo of The commercialization of 'Tepezza,' a targeted treatment for Thyroid Eye Disease, is expected. According to industry sources, the Ministry of Food and Drug Safety (MFDS) is reviewing the approval of the Thyroid Eye Disease (TED) treatment, Tepezza (teprotumumab). This drug was designated as an orphan drug in South Korea in August 2024. Tepezza is indicated for the 'treatment of adult patients with moderate to severe TED.' Tepezza is a monoclonal antibody that is designed to target insulin-like growth factor (IGF-1) receptor. It is an antibody drug administered once every three weeks (total of eight doses). Tepezza was approved by the US Food and Drug Administration (FDA) through the fast track program in January 2020. It was approved in Japan recently. This drug was initially developed by the Irish pharmaceutical company, Horizon Therapeutics. Then, Amgen acquired Horizon Therapeutics in 2022 and secured the sales right of the drug. The patients with TED who participated in the Tepezza clinical trial received an infusion of Tepezza or a placebo every three weeks for a total of eight infusions. In the OPTIC Phase 2 clinical trial, a total of 121 patients with chronic TED who have Clinical Activity Score (CAS) of greater than 4 and duration of illness under 9 months. Among the study participants, the number of patients of age greater than 40 was 99, and the average age was 54.5 years. Female participants over 40 accounted for 72.7% and diplopia was identified as 74.7%. The number of patients aged under 40 was 22, and the average age was 32.8 years. Female participants less than 40 accounted for 59.1%, and diplopia was identified in 68.2%. Based on the results analyzing the treatment response of Tepezza in chronic TED patients, the proportion of those who showed treatment response of improvements in exophthalmos by over 2 mm following 24-weeks treatment was 86.4% for the 40s or below group and 79.8% for the 40s or higher group. There was no statistical difference between these two groups. Meanwhile, Tepezza is regarded as having changed the treatment of TED. Before the introduction of Tepezza, steroids and orbital decompression surgery were the only options for the TED treatment. While steroids may reduce inflammation, they also pose concerns regarding adverse responses and the risk of relapse if patients discontinue the treatment. Orbital decompression surgery may result in complications.
- Company
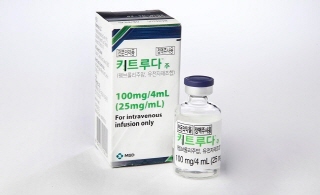
- Will Keytruda’s reimbursement standards be set this time?
- by Whang, byung-woo Feb 07, 2025 05:52am
- Keytruda, for which MSD had submitted reimbursement applications to extend its coverage to 17 indications, is gaining industry attention as it is expected to be presented to the Cancer Disease Deliberation Committee for the first time this year. Pic of Keytruda According to industry sources, MSD Korea's Keytruda (pembrolizumab) will be presented to the Korea Health Insurance Review and Assessment Service's Cancer Disease Deliberation Committee (CDDC) on the 12th. Keytruda received a redeliberation decision for the two indications of gastric cancer at the 9th CDDC meeting in December. There were hopes that a conclusion could be reached last October when MSD Korea submitted a new financial sharing proposal to expand coverage to 17 indications, including gastric cancer, but the year had passed with no solid results. In particular, there were various interpretations of the fact that only the 2 additional gastric cancer indications were discussed at the CDDC meeting, as the company applied for coverage of 17 indications. However, with Keytruda expected to be presented at the first CDDC meeting this year, industry eyes are on whether the tables will turn this time. Keytruda is currently approved in 33 indications for 17 different cancers and has applied for reimbursement for 17 of those indications. After applying for reimbursement for 13 indications in 2023, the company added four more indications last year: ▲ MSI-H gastric cancer, ▲MSI-H biliary tract cancer, ▲HER2-positive gastric cancer, and ▲HER2-negative gastric cancer. The issue is how CDDC will view MSD's proposed fiscal sharing plan. It is understood that the agency has asked MSD to provide additional data beyond its financial sharing plan. Given that the last review only discussed gastric cancer, it will be interesting to see how the government will regard the cost burden of applying the additional reimbursement to the 17 indications. At this point, it is unlikely that the CDDC will pass or fail review for individual indications. The CDDC had reviewed all indications at the same time, with the exception of the gastric cancer indication, which was added last December after MSD filed for Keytruda's reimbursement in bulk. This means that the decision is likely to be an all-or-nothing proposition. This has led some to speculate that MSD may take on a different strategy if Keytruda's reimbursement extension is not approved in this CDDC review. While there are advantages to applying for reimbursement extensions for many indications at once, the downside is that it is difficult to get feedback on individual indications. Considering how even if the application passes CDDC review, there are still procedures such as the Drug Reimbursement Evaluation Committee and the National Health Insurance Service’s drug pricing negotiations, MSD may well worry about the lack of progress if there is no change in its third year of application. “As it has been three years since MSD applied for the reimbursement extension, MSD would be contemplating on setting a specific strategy to overcome the current situation,” said an industry official. ”As MSD has stated that it submitted the reimbursement for multiple indications at once to not marginalize certain cancer types, I expect there will be discussions on various strategies depending on the results of the upcoming CDDC review.”
- Company
- Will the non-reimb status of 'Padcev' change this year?
- by Eo, Yun-Ho Feb 07, 2025 05:51am
- Product photo of PadcevThe industry is paying attention to the progress of reimbursing monotherapy·combination therapy of 'Padcev,' the new ADC drug for bladder cancer. Astellas Korea's Antibody-Drug Conjugates (ADC) Padcev (enfortumab) is expected to be considered for the Cancer Disease Review Committee of the Health Insurance Review and Assessment Service (HIRA) in February. At the end of last year, Astellas applied for reimbursement of Padcev for the monotherapy of adult patients with locally advanced or metastatic urothelial carcinoma who had previous experience with platinum-containing chemotherapy or PD-1 or PD-L1 inhibitors, and also applied for reimbursement of Padcev in combination with Keytruda (pembrolizumab), a PD-1 inhibitor, as a first-line treatment of advanced or metastatic urothelial carcinoma. The monotherapy passed the Cancer Disease Review Committee in February 2024 but after the economic evaluation, its reimbursement is being postponed due to disagreement between the government and the pharmaceutical company over the cost-effectiveness. Astellas plans to submit a supplement of the monotherapy while applying for reimbursement of the combination therapy. Padcev is recommended as a Category 1 preferred treatment option in the National Comprehensive Cancer Network (NCCN), and a novel treatment option for patients with urothelial cancer who have disease progression or relapsed following cancer immunotherapy and platinum-containing chemotherapy and are not eligible for previous standard-of-care treatment options. In South Korea, Padcev was approved in March 2023 as a monotherapy for patients with locally advanced or metastatic urothelial cancer who had previous experience with platinum-containing chemotherapy or PD-1 or PD-L1 inhibitors. Consequently, the industry is closely monitoring whether the status of Padcev, which remained a non-reimbursed drug for two years following its domestic approval, will change. Astellas representative stated, "Padcev combination therapy has changed the paradigm of the first-line treatment of metastatic urothelial carcinoma after 30 years and a demonstrated therapy with superior clinical benefits. We will strive to closely talk with the government and interested parties so that Korean patients with metastatic urothelial carcinoma receive benefits." The efficacy of Padcev monotherapy was demonstrated through the global EV-301 Phase 3 clinical study, which compared Padcev to existing chemotherapy in 608 patients with locally advanced or metastatic urothelial cancer who had previous experience with platinum-containing chemotherapy or PD-1 or PD-L1 inhibitors. The study results have shown that Padcev treatment lowered the death risk by approximately 30% compared to the existing chemotherapy, and the Padcev treatment group had a median overall survival (OS) of 12.9 months, significantly improving the OS compared to the 9.0 months of those treated with chemotherapy. The efficacy of Keytruda combination therapy was demonstrated through the EV-302 clinical study presented during the 2023 Congress of the European Society for Medical Oncology (ESMO Congress 2023). EV-302 is a randomized Phase 3 clinical study evaluating the effectiveness of Padcev+Keytruda combination therapy compared to platinum-based chemotherapy in 886 patients in 25 countries. Based on the study, at the median follow-up of 17.2 months, the patients had a median OS of 31.5 months, extending the OS by approximately twofold compared to the platinum-based chemotherapy group, and reduced death risk by 53%.
- Company
- CKD to distribute·sell Bayer's 'Nexavar'·'Stivarga' in KOR
- by Kim, Jin-Gu Feb 07, 2025 05:51am
- Product photo of Stivarga Chong Kun Dang Pharm announced on February 6 that it has signed an exclusive sales agreement with Bayer Korea for the advanced hepatocellular carcinoma treatments 'Nexavar (sorafenib)'·'Stivarga (regorafenib).' Based on this contract, Chong Kun Dang Pharm will be responsible for exclusive distribution·sales·marketing of Nexavar and Stivarga at hospitals and clinics, starting in February. Nexavar and Stivarga are targeted treatments for hepatocellular carcinoma. The effectiveness and safety of these drugs were demonstrated through various clinical trials. In 2018, insurance reimbursement for Stivarga was expanded, allowing it to be used as a second-line treatment for hepatocellular carcinoma in South Korea. Consequently, using Nexavar as a first-line treatment followed by Stivarga as a second-line treatment is now established as a reimbursable consecutive treatment option. Young-Joo Kim, CEO at Chong Kun Dang Pharm, said, "Chong Kun Dang Pharm has been enhancing its expertise in anticancer drugs, recently establishing a dedicated anticancer drug division." Kim added, "With a stronger portfolio that includes the exclusive distribution of Nexavar and Stivarga, we expect further to expand our presence in the domestic anticancer market." JinA Lee, CEO at Bayer Korea, said, "Based on long-established trust between both companies, we are pleased to distribute the Bayer products with Chong Kun Dang Pharm with distinguished competitiveness in the anticancer drug area." Lee added, "Based on the partnership, we hope to contribute to improving the quality of life of Korean patients with liver cancer by efficiently providing treatment options, such as Nexavar and Stivarga." Chong Kun Dang Pharm and Bayer Korea have been co-selling the antibiotics, 'Ciprobay' and 'Avelox' since 2005 and 'Kerendia,' a treatment for chronic kidney disease in adults with type 2 diabetes, since 2024. Also, they have established a successful partnership with Chong Kun Dang Pharm, which exclusively distributes Bayer Korea's 'Aspirin Protect' and 'Adalat OROS SR Tab,' which are treatments for cardiovascular diseases.