- LOGIN
- MemberShip
- 2025-12-21 17:44:02
- Company
- Hanmi partners with MSD for next-gen IL-2 analog development
- by Cha, Jihyun May 20, 2025 05:58am
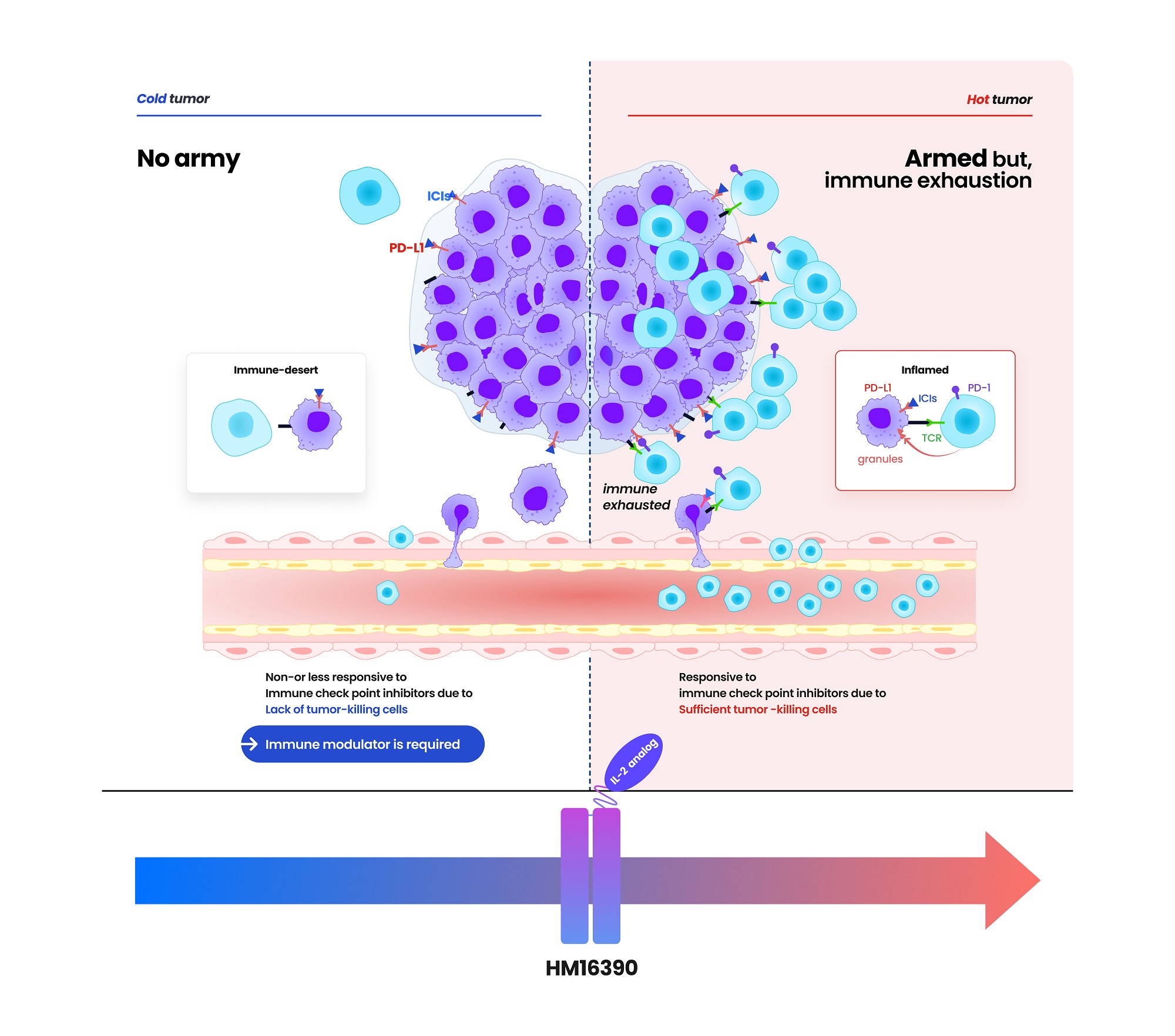
- Hanmi Pharmaceutical (CEO: Jae-Hyun Park) announced on the 19th that it has signed a clinical trial collaboration and supply agreement with U.S. Merck (MSD) to evaluate the combination therapy of its LAPS IL-2 analog 'HM16390' and MSD's anti-PD-1 immunotherapy 'Keytruda' (pembrolizumab). Hanmi Pharmaceutical will sponsor and oversee the Phase I clinical trial to evaluate the safety and efficacy of the combination therapy of HM16390 and Keytruda. MSD will supply Keytruda for the clinical trial. HM16390 is a next-generation IL-2 variant designed with a differentiated strategy that regulates the differentiation and proliferation of immune cells. HM16390 is designed to maximize antitumor effects by increasing the number of tumor-infiltrating lymphocytes that respond to immune checkpoint inhibitors in the tumor microenvironment through a mechanism that induces T cell proliferation and activation, thereby converting cold tumors with low immunogenicity into hot tumors with high immunogenicity. Currently approved recombinant IL-2 therapy 'Proleukin' is recommended for limited use due to side effect issues. Additionally, most IL-2 analogues in development focus on regulating the binding affinity of the IL-2 beta receptor, but this approach has shown limitations in terms of safety, according to Hanmi Pharmaceutical. Reducing the binding affinity of the IL-2 beta receptor decreases side effects such as vascular leak syndrome, but this also reduces anticancer effects. Conversely, increasing the binding affinity of the IL-2 beta receptor and eliminating binding with the IL-2 alpha receptor enhances anticancer effects, but this increases the risk of severe side effects such as cytokine release syndrome. To overcome these limitations, Hanmi Pharmaceutical has introduced a differentiated development strategy for HM16390. Unlike existing IL-2 candidates, HM16390 precisely regulates the binding affinity of the IL-2 alpha receptor, thereby ensuring safety while maximizing the efficacy of the drug. The company expects that this approach will maintain anticancer effects while minimizing serious side effects. (Data: Hanmi Pharmaceutical) HM16390 is an immunotherapy drug that maximizes the efficacy, safety, and durability by applying Hanmi Pharmaceutical's proprietary sustained-release platform technology, LAPSCOVERY. It is currently being developed as a sustained-release therapy that can be administered once per treatment cycle via subcutaneous injection (SC). Hanmi Pharmaceutical is developing HM16390 for use as a monotherapy and in combination with other immunotherapy agents for various solid tumors, and is currently conducting a global Phase I clinical trial. Dr. Jong Chul Park, Professor at the Massachusetts General Hospital (MGH) Head and Neck Cancer Center, Harvard Medical School, and the principal investigator for the Phase I clinical trial of HM16390 in Korea and the United States, said, “Through collaboration with MSD, we anticipate that the combination therapy of HM16390 and Keytruda will improve treatment outcomes for patients with advanced or metastatic solid tumors, and expect significant results in the future.” Young Su Noh, Director of Hanmi's ONCO Clinical Team, said, “Hanmi Pharmaceutical possesses a differentiated pipeline in the field of oncology, particularly in immunotherapy. We plan to sequentially showcase our research achievements through various academic conferences this year.”
- Company
- 'Oxlumo' for primary hyperoxaluria expected to be available
- by Eo, Yun-Ho May 19, 2025 05:56am
- The primary hyperoxaluria treatment, 'Oxlumo,' is expected to be commercialized in South Korea. According to sources, the Ministry of Food and Drug Safety (MFDS) reviewing Oxlumo (lumasiran) for approval. The MFDS granted 'Global Innovative products on Fast Track (GIFT)' designation to Oxlumo last year and orphan drug status in October of the same year. Oxlumo is an RNAi therapy for primary hyperoxaluria type 1 (PH1), a rare kidney disease, that was approved by the U.S. Food and Drug Administration (FDA), and the European Medicines Agency (EMA) in 2020. RNAi is one of the gene therapies considered a next-generation new drug technology, and it provides an advantage for specifically targeting human genes that cause diseases. PH1 is a rare disease in which the liver produces excessive oxalate. It causes the accumulation of oxalate crystals or calcium oxalate in the liver and urinary system. When the disease continues, the kidneys are damaged, requiring kidney dialysis. A treatment option for PH1 became available with the approval of Oxlumo in 2020. Oxlumo is an RNAi therapy targeting hydroxyacid oxidase 1 (HAO1), coding the oxalate-producing glycolate oxidase (GO) enzyme. It works by suppressing HAO1 and reducing GO production, ultimately reducing oxalate levels. Meanwhile, the efficacy of Oxlumo was found in a Phase 3 study involving 39 PH1 patients aged six years or older. Patients treated with Oxlumo had 65.4% lower oxalate levels in urine compared to the placebo group. Furthermore, 84% of patients treated with Oxlumo had oxalate levels close to normal. 52% of the group had recovered to the normal range.
- Company
- Hanmi 'Rolvedon' reports US sales gain ₩18B in Q1
- by Chon, Seung-Hyun May 19, 2025 05:55am
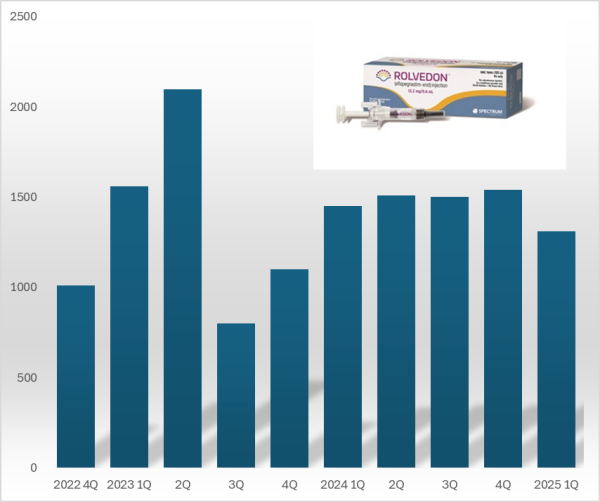
- 'Rolvedon,' a treatment for neutropenia that Hanmi Pharmaceutical licensed out, continues to be popular in the U.S. market. Although the growth trend has stalled due to price reduction in the U.S., Rolvedon recorded over US$ 10 million in sales for six consecutive years. Rolvedon's cumulative sales amounted to KRW 200 billion in two years and six months since the drug launched in the U.S. market. According to the reports by Assertio Holdings on May 16, Rolvedon's sales for Q1 amounted to US$13.10 million (KRW 18 billion). However, it decreased by 9.7% from US$14.50 million in Q1 and 14.9% from US$15.40 million in the previous quarter. The company explains that despite slightly reduced sales due to Rolvedon's price reduction, increased sales partially recovered lost sales from the price reduction. Rolvedon Rolvedon is a new biopharmaceutical that Hanmi Pharmaceutical licensed to Spectrum in 2012. It is administered to cancer patients receiving myelosuppressive chemotherapy for the treatment or prevention of neutropenia. It belongs to the 'G-CSF' (granulocyte colony-stimulating factor) class, which stimulates granulocytes to increase neutrophil counts, showing a mechanism of action similar to Amgen's blockbuster drug Neulasta (pegfilgrastim). In South Korea, Rolvedon received new drug approval from the Ministry of Food and Drug Safety in March 2021 under the product name 'Rolontis.' Spectrum was acquired by Assertio Holdings, a pharmaceutical company specializing in central nervous system disorders·pain·inflammation, in April 2023. Assertio Holdings is a pharmaceutical company specializing in developing CNS and inflammation treatments. The company has products such as the non-steroidal anti-inflammatory drug Indocin and buccal dissolving film Sympazan. The company has succeeded in strengthening its oncology pipeline by acquiring Spectrum. Assertio Holdings is responsible for clinical development, regulatory approval, manufacturing, and commercialization of Rolvedon and Poziotinib worldwide, excluding South Korea·China·Japan. Rolvedon recorded its first U.S. sales of US$ 10.1 million in the fourth quarter of 2022, generating revenue in the United States. In December 2022, Rolvedon was included in the prevention·treatment options guideline for febrile neutropenia of the U.S. National Comprehensive Cancer Network (NCCN). Rolvedon posted sales of US$ 15.6 million in the first quarter and US$ 21.0 million in the second quarters of 2023, but declined to US$ 8.0 million in the third quarter. It rebounded to US$ 11.0 million in the fourth quarter of 2023, surpassing US$ 10.0 million in sales for six consecutive quarters through the first quarter of this year. Rolvedon's cumulative U.S. sales reached US$ 138.8 million (KRW 194 billion). At the San Antonio Breast Cancer Symposium (SABCS 2024) held in the United States last December, Assertio Holdings released the Phase 1 clinical trial results of the same-day administration of Rolvedon. Existing neutropenia treatments, such as Neulasta, can only be administered 24 hours after chemotherapy. Administering a neutropenia treatment on the same day offers the advantage of potentially reducing patients' hospital stays. The clinical trial was conducted in 59 breast cancer patients, administering Rolvedon 30 minutes after chemotherapy to evaluate tolerability and safety. The trial found that the average time to neutrophil count recovery with Rolvedon was 1.8 days. Regarding safety, the adverse reactions observed with Rolvedon were similar to those reported in previous clinical studies. Assertio Holdings said, "Rolvedon's sales exceeded our internal forecasts despite securing inventory in the fourth quarter to support first-quarter sales growth," adding, "We expect Rolvedon's sales to continue rising due to strong ongoing demand."
- Company
- Ebglyss may be prescribed in general hospitals in Korea
- by Eo, Yun-Ho May 16, 2025 06:21am
- Pic of Ebglyss The new drug Ebglyss for atopic dermatitis may be prescribed in general hospitals in Korea. According to industry sources, Lilly Korea's interleukin (IL)-13 inhibitor Ebglyss (lebrikizumab) has passed the Drug Committees (DCs) of 9 medical institutions nationwide, including tertiary hospitals like Asan Medical Center and Sinchon Severance Hospital, as well as Seoul National University Bundang Hospital. Accordingly, if Ebglyss is successfully listed for insurance reimbursement, the drug is expected to quickly lead to prescriptions. Lilly accepted a price below the evaluated amount (below the weighted average price of substitute drugs) presented by the Drug Reimbursement Evaluation Committee of the Health Insurance Review and Assessment Service in February and is currently negotiating Ebglyss’s drug price with the National Health Insurance Service. If listed, there will be 7 treatment options available for atopic dermatitis in Korea: biological agents (injectables) “Dupixent (dupilumab)” and “Adtralza (tralokinumab);” and JAK inhibitors (oral) “Rinvoq (upadacitinib),” “Civinqo (abrocitinib),” and “Olumiant (baricitinib).” The health authorities have recently been considering whether to allow JAK inhibitors to be used in cases where patients do not respond adequately to existing treatments (biological agents) or have poor tolerability, which is expected to further intensify market competition. If approved, Ebglyss will immediately benefit from the regulatory changes. The drug was approved by the Ministry of Food and Drug Safety in August 2024 for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents 12 years of age and older (weighing at least 40 kilograms) who are inadequately controlled by topical treatments or for whom such treatments are not recommended. Ebglyss demonstrated its clinical efficacy and safety profile in a pivotal Phase III clinical trial. Patients who achieve a clinical response after 16 weeks of treatment can thereafter receive a maintenance dose (250 mg) every 4 weeks, making it a useful first-line treatment option for patients with atopic dermatitis in Korea. The clinical studies on which the license was based are the Phase III ADvocate-1, ADvocate-2, and ADhere trials. The trials evaluated the clinical efficacy and safety of Ebglyss in 1062 adults and adolescents with moderate-to-severe atopic dermatitis. In ADvocate-1 and ADvocate-2, which evaluated Ebglyss as a monotherapy, Ebglyss improved outcomes, with 58.8% and 52.1% (16.2% and 18.1%, respectively in the placebo arm) achieving Eczema Area and Severity Index (EASI) 75; and 38.3% and 30.7% (9% and 9.5%, respectively in the placebo arm) achieving EASI 90 during the induction period (weeks 0-16) compared to placebo. Also, after one year of maintenance therapy (Week 52), 81.7% of the Ebglyss arm achieved EASI 75 (vs. 66.4% in the placebo arm) and 66.4% achieved EASI 90 (vs. 41.9% in the placebo arm), demonstrating significant symptom improvement in the long term.
- Company
- AZ 'Imfinzi' leads the paradigm shift in cholangiocarcinoma
- by Whang, byung-woo May 16, 2025 06:18am
- "Introduction of Imfinzi in cholangiocarcinoma treatment can be seen as a critical advance. That a new therapy offering the possibility of long-term survival has appeared after 12 years is highly encouraging." As new treatment options for cholangiocarcinoma are introduced, a paradigm shift is said to be brought to this area, which was previously neglected and poorly developed. Although it is too early to be certain of long-term survival in South Korea, Dr. Yoo says that it could be a game-changer since new treatment options can benefit patient. Dr. Changhoon Yoo, Professor in the Department of Oncology at Asan Medical Center in SeoulDr. Changhoon Yoo, Professor in the Department of Oncology at Asan Medical Center in Seoul, who has expertise in this field, shared limitations in the treatment setting of cholangiocarcinoma and possible improvements. The prevalence of cholangiocarcinoma is known to be higher in Asia regions, including South Korea, China, and Taiwan, compared to Western countries. However, patients are often in advanced stages when diagnosed due to the challenging early diagnosis. It is one of the cancers that is difficult to reach a curative intent. Dr. Yoo explained, "Cholangiocarcinoma has a low prevalence due to its high mortality, resulting in a low cumulative patient number relative to its incidence. Currently, only about 20–30% of cholangiocarcinoma patients are eligible for surgery, and the remaining approximately 70% must rely on drug treatments such as chemotherapy or immunotherapy." While liver fluke infection was the leading cause of cholangiocarcinoma in the past, new factors such as fatty liver have emerged due to the westernization of dietary habits. 임핀지(더발루맙)After Imfinzi (durvalumab) received approval from the Ministry of Food and Drug Safety in November 2022, it is continuously expanding its influence. Dr. Yoo said, "Although less than three years have passed since the indication approval and it is therefore difficult to confirm long-term survival rates, in clinical practice the proportion of patients showing improvement has increased compared with before," adding, "Considering that patients who received Imfinzi combination therapy early in the 2021 clinical study still have favorable outcomes, Imfinzi can be seen as providing benefits to patients." In particular, Dr. Yoo focused on Imfinzi's side effects and safety. Dr. Yoo explained, "Most side effects are caused by the cytotoxic chemotherapy agents used in combination, and there are almost no issues attributable to Imfinzi. It rarely causes patients to struggle or reduces clinical efficacy, making it a medication that is of considerable help not only to patients but also to healthcare providers." "Korean subgroup analysis data on Imfinzi demonstrates long-term survival effects" Another reason why Imfinzi combination therapy is gaining attention in cholangiocarcinoma is that overall survival (OS) was shown to be higher in Korean patients. According to the study results, the two-year survival rate in the Korean patient group receiving the Imfinzi combination therapy was 38.5%, more than twice the 14.1% observed in the group that received chemotherapy. Furthermore, the survival rate at 36 months was 21.0% in the Imfinzi combination group, more than double the 8.8% in the chemotherapy group. Dr. Yoo analyzed, "In my opinion, I consider the Korean subgroup analysis data from the TOPAZ-1 study very encouraging. These results reflect the rapid accessibility and thorough patient management within the healthcare system." In cholangiocarcinoma, where inflammation or adverse reactions often occur during anticancer treatment, leading to treatment interruptions and repeated hospital admissions and discharges, continuous cancer treatment itself is challenging. According to Dr. Yoo, it is particularly common for treatment to be paused for a month or two due to inflammation, worsening the disease, and cholangitis can occur even when the cancer itself is not progressing. Therefore, high accessibility to treatment is critical in cholangiocarcinoma. Dr. Yoo said, "In Korea, if inflammation or jaundice occurs, patients can quickly visit a hospital, receive a procedure, and recover, thereby immune checkpoint inhibitors can be administered continuously," and added, "Compared to countries with less-established healthcare systems, our accessibility and level of care are higher, so I believe the effectiveness of immune checkpoint inhibitors can be more pronounced." Dr. Yoo also said, "Cholangiocarcinoma is indeed a challenging disease, but prognosis has improved recently and long-term survival cases are increasingly common," and added, "To secure approval or reimbursement for new drugs, one side's opinion is not enough. It is also necessary for patients and healthcare professionals to raise their voices together." Reimbursement discussions remaining for Imfinzi combination Therapy… "The standard criteria application should be avoided" However, the Imfinzi combination therapy is only reimbursed for the chemotherapy, and the cost barrier remains high. Currently, Imfinzi's reimbursement criteria were established in November of last year. Following AstraZeneca Korea's application for the cost‐effectiveness track, the Health Insurance Review and Assessment Service (HIRA)'s Economic Evaluation Committee is expected to discuss Imfinzi's cost‐effectiveness this month. According to industry sources, this month's Economic Evaluation Committee will review the cost‐effectiveness of Imfinzi+gem-cis combination therapy as a first‐line treatment for locally advanced or metastatic cholangiocarcinoma. It will be forwarded to the Drug Reimbursement Evaluation Committee if it passes the Economic Evaluation Committee. In this regard, Dr. Yoo noted that, for the sake of patient access, the standard criteria should be avoided. For example, in hepatocellular carcinoma, the standard treatment, sorafenib, is not particularly low‐cost, so its price could not be matched when a new drug emerged. Still, it is disadvantageous for a new drug to meet such price benchmarks for rare diseases or those where drug development has lagged. Dr. Yoo said, "When nanoliposomal irinotecan was introduced as a second‐line treatment for pancreatic cancer, it also faced challenges in economic evaluation when compared with 5‐FU." And added, "Likewise, I do not think conducting a straightforward economic comparison between existing cholangiocarcinoma drugs, whose patents have expired and thus are inexpensive, and an innovative new drug developed after a decade is appropriate." Dr. Yoo also described cholangiocarcinoma as 'the lung cancer of the gastrointestinal cancer family,' emphasizing the importance of precision medicine in new drug development. "Although cholangiocarcinoma has one of the poorest prognoses among gastrointestinal cancers, I am interested in the possibility of developing targeted therapies based on genetic analysis of specific biomarkers," Dr. Yoo added, "Approximately 4–5% of cholangiocarcinoma patients carry specific gene mutations, making this a cancer type with high potential for precision‐medicine application, and research is underway." Finally, Dr. Yoo urged, "Support for precision medicine and targeted‐therapy development is urgently needed to broaden patient treatment opportunities." And, "I hope that cholangiocarcinoma patients will not lose hope and will actively pursue their treatments."
- Company
- Doctors ‘Reimb too slow for new drugs in Korea’
- by Eo, Yun-Ho May 15, 2025 06:23am
- Most doctors were found to believe that the speed of reimbursement for new drugs in Korea is too slow. The Korean Research-based Pharmaceutical Industry Association (KRPIA) released the results of a survey of 100 domestic medical professionals on the 14th. In January, the global polling agency Ipsos Research surveyed domestic clinical experts from various medical departments to ask their opinions on access to new drugs. According to survey results, all medical professionals unanimously answered that the period from the Ministry of Food and Drug Safety approval to health insurance reimbursement listing is “long,” with 74% stating it is “too long.” Regarding the appropriate period from approval to health insurance listing, 81% of medical professionals answered “up to 10 months,” with 41% deeming “within 6 months” as appropriate. As of 2022, it takes an average of 608 days (approximately 20 months) for innovative new drugs to be approved by the MFDS and listed for health insurance reimbursement in Korea. This is twice the appropriate period cited by most medical professionals (10 months) and significantly longer than in major overseas countries such as Germany (281 days), Japan (301 days), and France (311 days) during the same period. Furthermore, experts directly treating patients in the clinical settings anticipate that the swift and widespread introduction of innovative new drugs will provide substantial benefits for patient care. Eighty-three percent of medical professionals expected that “if drugs already in common use overseas are covered by health insurance in Korea, patient treatment outcomes will improve significantly.” A large proportion of medical professionals (85%) responded that “even for drugs already covered by health insurance, if reimbursement standards are eased to enable early or wider use, patient treatment outcomes will improve significantly.” In addition, 95% of medical professionals urged the MOHW to introduce a “fast-track listing procedure or system” for health insurance coverage, similar to the MFDS's Global Innovate Products on Fast Track (GIFT) system, which shortens the drug approval review period for severe or life-threatening diseases by up to 75%. Medical professionals who participated in the survey also expressed concerns about Korea's low access to new drugs. Ninety-four percent of medical professionals pointed out that “Korea's access to new drugs is lower than overseas,” and 97% answered that “the government must set appropriate and reasonable drug prices to prevent the ‘Korea passing’ phenomenon, where multinational pharmaceutical companies give up the launch of innovative new drugs in Korea due to domestic regulations on pharmaceuticals.” Seventy-six percent of medical professionals were concerned that the proportion of new drug expenditures (13.5%) in total domestic drug expenditures is 60% lower than the OECD average (33.9%), and 88% believed that reimbursement and access to new drugs in South Korea need to be improved to the level of the top 10 OECD countries. Medical professionals identified “enhancing access to innovative new drugs” as the top priority among the four key strategies of the government's Second Comprehensive National Health Insurance Plan (2024-2028). As the government pushes policies to reduce drug costs in response to an aging society, 67% of medical professionals expressed the view that “the budget savings should be reinvested into the health insurance fund.” As the survey respondents were clinical experts, they also requested that the opinions of those working on-site be more actively taken into account in the reimbursement decision-making process. Eighty-eight percent of medical professionals responded that “the opinions of medical professionals should be better reflected in the process of registering drugs for health insurance coverage,” and 80% said that “medical professionals should also be involved in the process of selecting patient population eligible for health insurance coverage.” A KRPIA official stated, “Medical professionals who care for patients on the front lines are deeply concerned about the difficulties patients face in receiving treatment due to delays in the introduction of innovative new drugs. They hope that new drugs will be listed for health insurance reimbursement more swiftly and with a broader scope. We anticipate that the results of this survey will contribute to the government’s fostering of a patient-centered treatment environment and policy design.”
- Company
- Arexvy opening the era of RSV vaccine
- by Whang, byung-woo May 15, 2025 06:22am
- As GSK launches the respiratory syncytial virus (RSV) vaccine Arexvy in South Korea, it will challenge the market on a full-scale. Arexvy is already expanding its market dominance in the global market with its strength as the first RSV vaccine. The company will likely focus on expanding vaccine awareness as it opens the RSV vaccine market for the first time. Dr. Ji-Yong Moon, Professor of Konkuk University On May 14, GSK Korea convened a press conference celebrating the launch of Arexvy, the world's first RSV vaccine. The company showcased a preventative strategy for seniors against RSV and Arexvy's clinical significance. Arexvy received approval from the Ministry of Food and Drug Safety (MFDS) at the end of December 2024 for the 'Prevention of lower respiratory tract disease (LRTD) caused by RSV in adults over 60 years of age and older.' Approval of Arexvy was based on results from two Phase 3 studies, 'RSV OA=ADJ-006' and 'RSV OA=ADJ-004,' involving adults 60 years of age and older. The study results showed that during the first RSV season, Arexvy significantly lowered the RSV-LRTD risk by 82.6% and severe RSV-LRTD risk by 94.1% in participants 60 years of age and older compared to placebo. Furthermore, the efficacy of the vaccine regarding RSV-A-associated LRTD increases and RSV-B-associated LRTD increases were 84.6% and 80.9%, respectively. Dr. Ji-Yong Moon, Professor of Konkuk University's Department of Respiratory-Allergy and Clinical Immunology, explained, "RSV infection causes complications, such as pneumonia, in adults aged 60 years or older and it may require hospitalization or lead to death in severe cases," and added, "Based on a retrospective study, 56.8% of the hospitalized adults over age of 65 had pneumonia and 10.6% of those died." Dr. Moon added, "Despite the high disease burden, awareness of RSV infection is poor, and differential testing is not well implemented, so the disease burden of RSV infection has been underestimated." He said, "RSV infection is as contagious as influenza, but there is no specific treatment other than supportive care, so prevention is most important." (from left) Professor Jacob Lee, Professor of the Division of Infectious Disease at Hallym University Kangnam Sacred Heart Hospital, and, Dr. Ji-Yong Moon, Professor of Konkuk University Experts consider that the launch of the first RSV vaccine, Arexvy, is expected to be significant from a preventive standpoint. Professor Jacob Lee, Professor of the Division of Infectious Disease at Hallym University Kangnam Sacred Heart Hospital, reported that RSV vaccination is already recommended in the United States. Dr. Lee said, "Arexvy showed a preliminary efficacy of 94.6% against RSV-LRTD in adults with one or more comorbidities," and emphasized, "Considering that 84% of domestic adults aged 65 and over have one or more chronic diseases, these data are noteworthy." Dr. Lee stated, "Arexvy was approved in the U.S. in 2023, real-world data on its use have accumulated, and excellent preventive efficacy has been confirmed in actual clinical settings." He said, "The U.S. Advisory Committee on Immunization Practices (ACIP) recommends RSV vaccination for high-risk individuals aged 60–74 and all adults aged 75 and older." Cost and awareness remain challenges… "NIP is necessary from a long-term perspective" Regardless of the preventive value of RSV through Arexvy, it remains uncertain how much influence it will exert in the market. Currently, Arexvy remains non-reimbursed in South Korea. Without a recommendation like that of the U.S. ACIP, there is a need to improve awareness. In particular, the fact that the recommended vaccination age in the U.S. is higher at 75 years old, compared to the domestic approval age of 60 and over, also raises questions. Hyunji Kwon, Business Unit Head at GSKRegarding this, Dr. Lee explained, "In South Korea, the Korean Society of Infectious Diseases is expected to announce a recommendation within this year, but it is unlikely to differ significantly from the U.S.," and added, "To enter the National Immunization Program (NIP), sufficient disease burden research needs to be conducted." Dr. Lee continued, "There is a cost burden and domestic data are lacking, but as the population structure changes, the number of vaccination targets will also increase. Through research data and cost-effectiveness, we can hope for RSV prevention through the NIP in the long term." GSK Korea has stated that it will work to improve awareness through TV advertisements and other means alongside the launch of Arexvy. Hyunji Kwon, Business Unit Head at GSK, added, "RSV is a disease with a great unmet medical need yet remains unfamiliar, so we will work to improve awareness among medical staff and high-risk patients," and added, "In a super-aged society in which, for the first time, the population in their 60s exceeds that in their 40s, we will collaborate with the medical community and the government to increase patient access."
- Company
- Will a new trend emerge for liver cancer treatment?
- by Moon, sung-ho May 15, 2025 06:22am
- With new anticancer drugs entering the liver cancer treatment market, where combination therapy has been gaining prominence, attention is focused on whether a paradigm shift will occur. This is because a new competitive landscape is forming with the arrival of newly approved drugs and newly reimbursed drugs. # According to industry sources on the 7th, the US Food and Drug Administration (FDA) recently approved the combination therapy of Bristol Myers Squibb (BMS) and Ono Pharmaceutical's Opdivo (nivolumab)+Yervoy (ipilimumab) combination as a first-line treatment for adult patients with unresectable or metastatic hepatocellular carcinoma (HCC). The approval was based on the results of a randomized, open-label, global Phase III clinical trial (CheckMate-9DW) comparing the combination therapy of Opdivo and Yervoy (335 patients) with either lenvatinib or sorafenib monotherapy (333 patients). The trial was conducted on patients with unresectable or metastatic HCC who had not previously received systemic therapy. The CheckMate-9DW results showed that the median overall survival (mOS) in the Opdivo+Yervoy combination therapy group was 23.7 months (95% CI: 18.8-29.4), compared to 20. 6 months (95% CI: 17.5-22.5) in the control group (n=333), reducing the risk of death by 21% (HR=0.79; P=0.0180). In addition, the 3-year survival rate was 38% in the Opdivo+Yervoy combination therapy group, higher than the 24% in the control group. The objective response rate (ORR) was also significantly higher in the combination therapy group at 36.1% (95% CI: 31–41.5) compared to 13.2% (95% CI: 9.8–17.3; P
- Company
- 'Adempas' for pulmonary hypertension closer to obtain reimb
- by Eo, Yun-Ho May 14, 2025 06:11am
- Product photo of Adempas A new reimbursable treatment option for pulmonary hypertension is anticipated to be introduced. According to industry sources, Bayer Korea has finally reached an agreement with the National Health Insurance Service (NHIS) for its 'Adempas (riociguat).' Accordingly, after 10 years since it was approved in South Korea, Adempas will likely be included in the reimbursement list. Adempas' negotiation was a dosage negotiation, rather than a ceiling price negotiation. Bayer accepted the price 100% below the weighted average price (WAP) of a substitute drug and passed the Drug Reimbursement Evaluation Committee (DREC) of the Health Insurance Review and Assessment Service (HIRA) in February. Adempas was exempted from the drug pricing negotiations. This drug obtained approval in South Korea as an orphan drug in June 2014. Five products with different doses are available, and it has the efficacy and the effect in ▲patients with persistent/recurrent chronic-thromboembolic pulmonary hypertension (CTEPH, WHO Group 4) after surgical treatment or inoperable CTEPH, to improve exercise capacity ▲adult patients with arterial pulmonary hypertension (PAH, WHO Group 1) who have WHO functional class 2-3, to improve exercise capacity. Adempas has been known as the first novel drug to treat CTEPH. CTEPH occurs in patients with chronic pulmonary embolism who progress to chronic obstructive pulmonary disease (COPD) and develop fibrotic stenosis and occlusion, leading to pathological vascular remodeling and increased resistance in the pulmonary arteries. CTEPH is a chronic disease that causes progressive shortness of breath and right heart failure. Symptoms include dyspnea, fatigue, chest pain, dizziness, peripheral edema, cough, and hemoptysis, significantly impacting quality of life. Ultimately, it can progress to heart, kidney, and liver failure, potentially leading to death. Meanwhile, Adempas is a stimulator of soluble guanylate cyclase (sGC), an enzyme found in cardiovascular organs. The efficacy of the drug in patients with chronic thromboembolic pulmonary hypertension (CTEPH) was confirmed in Phase 2 and Phase 3 clinical trials. The clinical trial results showed that Adempas improved the study's primary endpoint physical activity and demonstrated superior tolerability. No unusual adverse reactions were reported. In the CHEST-1 study, results from comparing the 6 Minute Walking Test (6MWT) at 16 weeks from baseline showed that the patient group treated with riociguat had statistically significant improvement compared to the placebo group. In the PATENT-1 study, comparison of changes in 6MWT values at 12 weeks to the placebo group demonstrated statistically significant improvement, meeting the primary endpoint.
- Company
- U.S. executive order on drug price cuts raises hope
- by Kim, Jin-Gu May 14, 2025 06:10am
- U.S. President Donald Trump has signed an executive order to significantly reduce drug prices in the United States. This measure, which focuses on significantly reducing U.S. drug prices in line with overseas prices, is expected to have a positive effect on domestic biosimilars. However, some predict that the expected effects of this executive order will not be sufficient and that there will be no significant impact. Trump signs executive order to lower drug prices in the US... “Up to 90% reduction” According to local media reports on the 13th, President Trump signed an executive order on the 12th (local time) to lower the prices of prescription drugs in the US to the same level as other countries. The order essentially applies the most-favored-nation policy to drug prices in the US. Under the executive order, the US Secretary of Health and Human Services must promote a program that allows US patients to purchase drugs directly from pharmaceutical companies at a “most-favored-nation” price. In addition, the Secretary of Health and Human Services must impose the most-favored-nation pricing on companies within the US pharmaceutical industry within 30 days. This measure is interpreted as a move made to present a kind of price cap to pharmaceutical companies compared to drug prices overseas, and pressure them to lower their prices accordingly. Ultimately, this is expected to improve the intermediate distribution structure of Pharmacy Benefit Managers(PBMs) and further reduce the prices of expensive drugs. President Trump said at a press conference, “What we are trying to do is level the playing field for drug prices,” adding, “The American people will be able to purchase drugs at the lowest prices in the world.” He did not specify which drugs would be subject to price reductions. However, the administration has hinted that the price reduction could be as high as 90%. President Trump said at the press conference, “Drug prices in the United States could be reduced by 59%, 80%, or even 90%.” A day earlier, he had posted on his Truth Social account, “Prescription Drug and Pharmaceutical prices will be REDUCED, almost immediately, by 30% to 80%.” “Original drug price cuts expected to expand market for Korean biosimilars” The domestic pharmaceutical and biotech industry has responded with more optimism than concern to these measures. There are expectations that the price cuts targeting original drugs will have a positive impact on biosimilars. Celltrion said it expects the measure to create “a better business environment.” With the simplification of the intermediate distribution structure, it is expected that “this will provide positive opportunities for Celltrion's U.S. business activities,” and that “the dominance of pharmaceutical companies making high profit selling original products is expected to weaken, which could present market expansion opportunities for biosimilar companies.” Furthermore, it is anticipated that “biosimilar manufacturers will be able to negotiate drug prices directly with the government rather than through intermediaries such as PBMs, which could benefit both the government and manufacturers.” Additionally, it predicted that the prescription of biosimilars will increase as the prices of high-priced drugs are reduced. Currently in the US, through insurance companies and PBM systems, high-priced original drugs are prioritized for inclusion in formularies, followed by limited competition among biosimilar products, resulting in the addition of 2–3 products. During this process, rebates are paid to intermediary distributors, so the burden associated with these rebates will be significantly reduced with the new system. Celltrion stated, “Previously, biosimilar prices were set at the same high level as originals, making it impossible to provide substantial benefits to patients. However, if the intermediate distribution structure is improved through this executive order, the actual prescription prices of biosimilars will decrease, ultimately expanding biosimilar prescriptions to European levels.” Furthermore, it is also expected to provide an opportunity to launch new products in the US market. Celltrion explained, “If parallel imports are activated to supply medicines at most-favored-nation prices in accordance with this executive order, Celltrion will secure the opportunity to launch additional products that have not yet been introduced in the US market.” Another biosimilar company, Samsung Bioepis, is said to have shown a similar response. A positive outlook on the newly announced measure is dominant both inside and outside the company. The background is the expectation that the preferential policy for biosimilars will be strengthened to reduce medical costs. However, a Samsung Bioepis official said, “We are closely monitoring this policy,” without further elaboration. “It is premature to make specific judgements… Its impact on the Korean pharmaceutical industry will be limited The key issue is the strong opposition from the U.S. pharmaceutical and biotech industry. The U.S. pharmaceutical and biotech industry has consistently opposed the U.S. government's repeated attempts to lower drug prices, either by blocking them altogether or minimizing their impact. As a result, the actual evaluated effect of the drug price reductions was then minimal. In fact, President Trump signed an executive order for drug price cuts during his first term in 2018, but it was ultimately scrapped due to opposition from the pharmaceutical industry. At the time, an attempt was made to lower drug prices through an international reference pricing system, but a federal court raised procedural issues and put the brakes on the plan. President Joe Biden also conducted drug price negotiations with major pharmaceutical companies under the Inflation Reduction Act (IRA). However, the actual drug price reductions were limited to 10 drugs, including Eliquis, Xarelto, Januvia, Forxiga, Entresto, Enbrel, Imbruvica, Stelara, and Fiasp. Even these are limited to those who have Medicare - people aged 65 and older - in the United States. The drug price reduction measures will take effect in 2026, and the annual savings in medical costs are estimated at USD 6 billion (approximately KRW 8 trillion). This is considered insignificant compared to the total drug costs in the United States, which amounted to USD 805.9 billion (approximately KRW 1,142 trillion) last year. In this situation, the Trump administration's push for more stringent drug price reduction policies in the second term is expected to face strong opposition from the U.S. pharmaceutical industry. Additionally, criticism has emerged locally that the executive order lacks specific policies. The New York Times (NYT) criticized the executive order, stating that it “did not include specific policies such as pushing for legislation to reduce drug prices or revising drug payment regulations under government health programs.” In the same line, there are also projections that its impact on the domestic pharmaceutical and biotech industry will be limited. A representative from the pharmaceutical industry stated, “So far, only the direction to pursue drug price cuts has been outlined; there are no specific methods for how the cuts will be implemented, nor have the target products been determined.” The official added, “Its impact on domestic pharmaceutical and biotech companies is currently unclear, and even if there is an impact, it is expected to be minimal.” Another industry insider noted, “The recent administrative order requiring the manufacture of pharmaceuticals within the United States, as well as the upcoming pharmaceutical tariff policy expected next week, are likely to have a greater impact on the domestic pharmaceutical and biotech industry than the recent drug price reduction administrative order.”