- LOGIN
- MemberShip
- 2025-12-19 17:27:20
- Policy
- Introduction of AI for drug approval and review in Korea
- by Lee, Hye-Kyung Jul 10, 2025 06:09am
- The Ministry of Food and Drug Safety is conducting follow-up research to introduce generative artificial intelligence (AI) to the domestic drug approval and review field starting next year. The Pharmaceutical and Medical Device Research Department of the National Institute of Food and Drug Safety Evaluation is conducting an ISP project to establish the system, and plans to gradually expand the scope of AI application from chemical drugs to biopharmaceuticals. The Pharmaceutical and Medical Device Research Department of the National Institute of Food and Drug Safety Evaluation met with MFDS press corp reporters at the Jeju National Herbal Resource Management Center presented initiatives including research using AI and big data, the development of impurity analysis methods for pharmaceuticals, evaluation technologies for biological products, and plans to distribute standardized herbal reference substances. (From the left) Kyung-hoon Son, Director of the Drug Research Division;, Cheol-hyun Lee, Director of the Biologics Research Division; Gidae Park, Acting Director, Division of Advanced Biopharmaceutical Research; Jinhee Hwang, Director, Herbal Medicine Research Division; Youngmi Song, Director, Cosmetics Research Division; Haedae Park, Director, Division of Medical Device Research On the day, Jiwon Jeong, Director-General of the Pharmaceutical and Medical Device Research Department, opened the meeting with an explanation of the current status of research related to artificial intelligence and big data. Jeong said that new projects being promoted this year include research on strategies for utilizing AI in drug reviews. Director-General Jeong explained, “It is a two-track approach: the AI system is being developed by the IT team, while we are researching to determine whether AI can be used in medical product reviews.” She added, “The process of introducing AI into medical product reviews requires an enormous amount of data, so it is difficult to initiate everything at once. Starting next year, we will implement the project in phases, beginning with the simple and repetitive task of preparing review materials. We will start with chemical drugs.” Kyung-hoon Son, Director of the Drug Research Division, then discussed research on utilizing big data in the course of developing technology for assessing the safety of overseas manufacturing facilities related to Good Manufacturing Practice (GMP) standards. Director Son explained, “We conduct on-site inspections of overseas manufacturing facilities every year, but since each facility has its own system, there are challenges in organizing the data. Through this research, we aim to develop technology to standardize data related to GMP to assist with overseas inspections.” He also noted, “This research utilizing big data has not yet progressed to the stage of using AI. Even without collaborating with overseas companies, we believe it will be possible to develop and utilize a model that utilizes big data domestically.” In addition, the Pharmaceutical and Medical Device Research Department is promoting research on the development of specialized translation models (Korean-English) for safety management in the field of pharmaceuticals, the application of next-generation advanced pharmaceutical innovation technologies such as AI to manufacturing facilities, and a model for the utilization and dissemination of public data based on adverse drug reaction reports. (from the left) Jiwon Jeong, Director-General of the Pharmaceutical and Medical Device Research Department, Kyung-hoon Son, Director of the Drug Research Division, Cheol-hyun Lee, Director of the Biologics Research Division ◆ Research on impurity analysis methods expanded to include new substances=The National Institute of Food and Drug Safety Evaluation is also conducting research on the safety management of impurities and infectious diseases in pharmaceuticals. This includes developing and providing impurity analysis methods, developing and publishing impurity safety management guidelines, and compiling and distributing case studies on impurity occurrence assessments. In this regard, Director Son said, “The current research focuses on how to reduce single impurities such as N-nitrosodimethylamine (NDMA),” adding, “We are also conducting research on simultaneous analysis methods in consideration of small-sized companies.” He emphasized, “In particular, since the beginning of this year, we have been analyzing and categorizing the characteristics of newly developed substances, not just single substances, to develop analysis methods. Although the results are not yet available, we are striving to manage impurities that did not exist before.” The development of evaluation technologies for biological products is a research area currently being pursued by the National Institute of Food and Drug Safety Evaluation to strengthen infectious disease control. Biological products include mRNA vaccines, antibody-drug conjugates (ADCs), and antibody-based therapeutics such as monoclonal antibodies, bispecific antibodies, and small-molecule–multi-specific antibody combinations. Cheol-hyun Lee, Director of the Biologics Research Division, explained, “We have been conducting research on delivery methods and materials for mRNA vaccines for about 5 years, and we expect to complete the research this year.” He added, “We have not developed evaluation technologies for specific products or indications in the field of antibody drugs, but are creating relevant guidelines and information booklets. We are indirectly supporting companies' relevant research and development through methods such as introducing overseas cases.” Jeju National Herbal Resource Management Center ◆ National Herbal Resource Management Center owns 396 standardized herbal reference materials = The National Institute of Food and Drug Safety Evaluation is also creating standard herbal medicine products through the National Herbal Resource Management Center. Located in three locations, Yanggu-gun, Gangwon-do; Okcheon-gun, Chungcheongbuk-do; and Seogwipo-si, Jeju-do, the National Herbal Resource Management Center serves as a control tower for the preservation, research, and investigation of herbal medicine resources in Korea. According to data from the National Institute of Food and Drug Safety Evaluation, the National Herbal Resource Management Center currently has 396 standardized herbal reference materials, including 273 standard herbal items, 120 marker compounds, and 3 reference materials. This is an increase of 12 items compared to 384 in 2023. Jin-hee Hwang, Director of the Herbal Medicine Research Division, said, “About five standard products are added every year. Although no products have been successfully commercialized, research using standard products is ongoing at Jeju Techno Park and Jeju University laboratories.” Additionally, the distribution of standardized herbal reference materials will not be categorized by sector such as pharmaceuticals, health functional foods, or cosmetics, but rather based on their intended use, such as quality control or research and development. The National Herbal Resource Management Center is currently working to improve the distribution system and plans to implement sector-based distribution, including for pharmaceuticals, starting next year.
- Policy
- Daewoong’s generic version of Migbose approved in Korea
- by Lee, Hye-Kyung Jul 09, 2025 06:09am
- Daewoong Pharmaceutical has been approved to sell a generic version of the diabetes treatment Migbose Film-Coated Tablets (miglitol) in Korea. On the 7th, the Ministry of Food and Drug Safety approved Daewoong Pharmaceutical's Daewoong Miglitol Tab. (miglitol). Like the original Migbose, Daewoong Miglitol Tab is a film-coated tablet indicated for the treatment of non-insulin-dependent diabetes mellitus (NIDDM) whose hyperglycemia cannot be adequately controlled by diet alone or with diet in combination with sulfonylurea therapy. It should be taken once daily with a small amount of water before meals or with a small meal. Although the active ingredient was developed as a diabetes treatment, it is primarily being prescribed off-label in practice for weight management. Approved in 2013, Migbose has a structure most similar to glucose, which stimulates the release of GLP-1 (glucagon-like peptide) and improves gastrointestinal side effects. Migbose regulates blood sugar by inducing the secretion of GLP-1. GLP-1 promotes the secretion of insulin, which lowers blood sugar, and inhibits the secretion of glucagon, which accumulates glucose and raises blood sugar, thereby regulating blood sugar levels. Migbose induces clinically significant levels of GLP-1 release upon intake, thereby regulating blood sugar levels. It also increases satiety by reducing gastrointestinal motility, making it useful for diabetic patients who need to lose weight. In addition, it is absorbed gradually in the small intestine, reducing the amount of carbohydrates that enter the large intestine, and therefore has relatively few gastrointestinal side effects. Daewon Pharmaceutical transferred the rights to Migbose to Daehan New Pharm in 2020. According to the pharmaceutical research institute IQVIA, the outpatient prescription amount for Migbose increased from KRW 0.5 billion in 2022 to KRW 1.2 billion in 2023.
- Policy
- Industry requests postponement of drug reimbursement reevals
- by Lee, Tak-Sun Jul 08, 2025 06:37am
- The pharmaceutical industry has expressed the need to postpone next year's drug reimbursement reevaluations to the government. The reason is that the reevaluation targets have not been decided by the second half of the year, leaving insufficient time for the target companies to prepare the necessary data. As a result, some are even suggesting that the reevaluations be skipped next year. According to industry sources on the 7th, the Health Insurance Review and Assessment Service held a meeting with pharmaceutical organizations, including the Korea Pharmaceutical and Bio-Pharma Manufacturers Association, on the 4th to discuss the drug reimbursement adequacy reevaluations for 2026 and thereafter. The first phase of the drug reimbursement adequacy reevaluations will be completed this year. A pilot project was launched in 2020 targeting choline alfoscerate, for which reevaluations have been ongoing for 6 years. The ingredients subject to reevaluation were drugs that were listed for reimbursement from 2006 to 1990, before the implementation of the positive listing system. Accordingly, it is expected that the second phase of reimbursement reevaluation will focus on ingredients registered after the positive listing system was implemented. In fact, in a study on “Measures to Rationalize the Drug Reimbursement Adequacy Reevaluations” that was conducted by HIRA, the researchers presented a plan for the second phase of reimbursement adequacy reevaluations, focusing on ingredients registered between 2007 and 2013. However, it is known that some government officials are planning to strengthen the selection criteria and re-examine products that were registered before the positive listing system was introduced. The current criteria for drugs with annual claims of KRW 20 billion or more may be lowered to KRW 10 billion or more, and drugs whose reimbursement adequacy was controversial will be selected. However, as discussions with the pharmaceutical industry have only just begun, it is expected to take more time before the target ingredients are finalized. The pharmaceutical industry believes that it will be difficult to conduct a normal drug reimbursement adequacy reevaluation next year due to delays in discussions on target ingredients. An industry official said, “In the first phase, the ingredients to be reviewed for the following year were announced in March of the previous year, but this year, the ingredients have not been decided even after the start of the second half of the year, so it will be difficult to prepare data. The industry hopes that the data submission period will be extended or that the review will be postponed until the year after the next.” The ingredients to be reviewed for reimbursement adequacy in 2025 were announced in March last year. These opinions were delivered to HIRA by industry representatives at a meeting held on the 4th. Meanwhile, the first results of this year's reimbursement adequacy reevaluation will likely be announced after the DREC meeting in August. This year's reevaluation includes domestically produced natural drugs such as styrene and Joins, and the industry is paying close attention to the first results.
- Policy
- Low-strength salt-modified Pristiq now available
- by Lee, Tak-Sun Jul 08, 2025 06:35am
- Companies with salt-modified formulations of the antidepressant Pristiq (desvenlafaxine succinate monohydrate) are strengthening their market competitiveness with a 25mg low-strength product that had not been available in the original or generic formulations. With price adjustments near due to the entry of generics, attention is focused on whether the release of these salt-modified formulations will bring success. According to industry sources on the 6th, three items, including Nexpharm Korea's Desvela SR Tab 25mg, Myung In Pharmaceutical's S-Ven ER Tab 25mg, and Han Lim Pharm’s Prinexor ER Tab 25mg, were listed for reimbursement this month. The active ingredient in these products is desvenlafaxine benzoate, and the drugs are salt-modified versions of the original Pristiq. Neither the original nor the generic products have been available in a 25mg dosage until now. Prior to this, Whan In Pharm launched Defaxine SR Tab 25mg (desvenlafaxine) in June 2022. When patients suddenly discontinue taking antidepressants, withdrawal symptoms such as nausea, dizziness, anxiety, and other adverse reactions may occur. Therefore, a gradual reduction in dosage is necessary. In this context, the 25mg low-dose formulation of desvenlafaxine is expected to be useful for gradual dosage reduction. With a year passing since the launch of generic versions of desvenlafaxine benzoate preparations, drug price adjustments are scheduled for August this year to a level of 53.55%. As a result, drug prices for salt-modified drugs that have received premium prices may also be reduced. The 25 mg desvenlafaxine benzoate preparation, which has been added to the reimbursement list, is also scheduled to undergo a drug price adjustment one month later. With price adjustments imminent, companies need to preoccupy the market at its current price in just a month. The current maximum prices are KRW 469 for Nexpharm Korea's Nexpharm Korea's Desvela SR Tab 25mg, KRW 468 for Myung In Pharmaceutical's S-Ven ER Tab 25mg, and KRW 450 for Han Lim Pharm’s Prinexor ER Tab 25mg. Hanlim voluntarily lowered its price below the assessed price. A month later, the price of Desvela SR Tab 25mg will be adjusted to KRW 359, and the other 2 products will be priced similar at KRW 358. Last year, based on UBIST data, the three brands recorded the following outpatient prescription sales: Myung In Pharmaceutical's S-Ven ER Tab KRW 11.2976 billion, Nexpharm Korea's Desvela SR Tab KRW 50,981 million, and Han Lim Pharm’s Prinexor KRW 393.8 million. The original Pristiq SR TAb recorded KRW 2.2 billion, and Whan In Pharm launched Defaxine SR TAb recorded KRW 1.6 billion, and is leading the market.
- Policy
- Boryung's follow-on Lenvima, 'Lenvanib,' becomes reimbursed
- by Lee, Tak-Sun Jul 07, 2025 06:09am
- Product photo of LenvimaBoryung has successfully obtained reimbursement listing for all dosages of 'Lenvanib Cap (lenvatinib mesylate dimethyl sulfoxide),' its follow-on drug to the anti-cancer medication Lenvima (lenvatinib mesylate, Eisai), which is used to treat conditions such as liver cancer. With the patent dispute with the original manufacturer, Eisai, still ongoing, Boryung is expected to commence sales following the listing of reimbursement. According to industry sources on July 4, Boryung's Lenvanib Cap 10 mg and Lenvanib Cap 12 mg were listed for reimbursement this month. Consequently, all three approved products, including Lenvanib Cap 4mg, which was listed for reimbursement in May, can be reimbursed. Lenvanib Cap is the first follow-on drug to Eisai's Lenvima in Korea. Unlike Lenvima, it has a solvate (dimethyl sulfoxide) attached. Boryung proved its equivalence to Lenvima through bioequivalence testing. Through this, Boryung filed patent invalidation or circumvention, and most of its claims were accepted by the Intellectual Property Trial and Appeal Board. However, Eisai has appealed the rulings concerning the invalidation of the use patent and the circumvention of the composition patent, and these disputes are currently ongoing at the Intellectual Property Court of Korea. The drug price for Lenvanib Cap 4mg and Lenvanib Cap 10mg is the same at KRW 26,765, while Lenvanib Cap 12mg is KRW 29,442, making them slightly cheaper than the original drug. The original product, Lenvima Capsule 4mg and 10mg, costs KRW 29,739. There is no 12mg product for the original drug. The approved indications for both products are identical, covering a total of four efficacies·effects, ▲Locally recurrent or metastatic progressive differentiated thyroid cancer refractory to radioactive iodine ▲First-line treatment of unresectable hepatocellular carcinoma (HCC) ▲Combination therapy with pembrolizumab for the treatment of advanced endometrial carcinoma that is not MSI-H (microsatellite instability high) or dMMR (mismatch repair deficient), in patients who have received prior systemic therapy and whose disease has progressed, and for whom surgical or radiation therapy is not suitable ▲First-line treatment of advanced renal cell carcinoma in combination with pembrolizumab. Among these, the two companies are disputing over the use patent related to thyroid cancer. The substance patent expired in April. As Boryung has successfully obtained reimbursement listing for all three dosages of its follow-on drug to Lenvima, it is expected to proceed with sales based on the Intellectual Property Trial and Appeal Board's ruling. However, an analysis suggests that Boryung is likely to proceed cautiously with sales activities until the court results are finalized, as a loss in the ongoing patent litigation at the Intellectual Property Court of Korea could lead to market withdrawal and the risk of patent infringement compensation. The original Lenvima recorded sales of KRW 10.3 billion in 2023, based on IQVIA data.
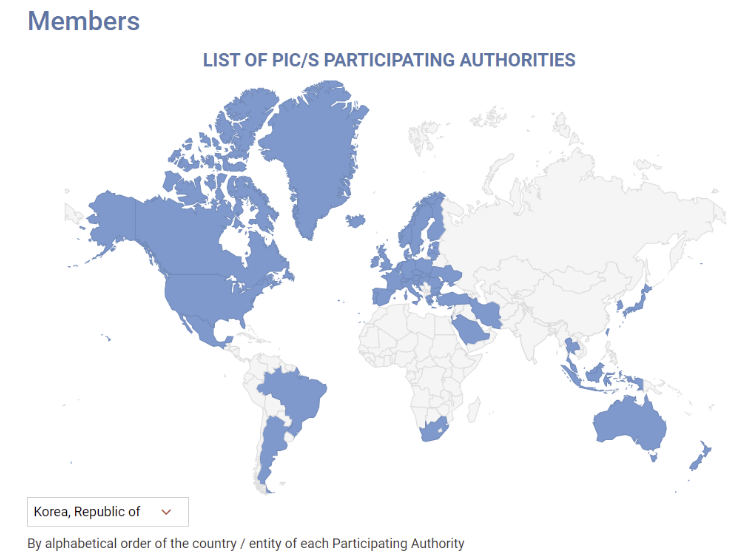
- Policy
- MFDS reviews GMP standards for sterile preparations abroad
- by Lee, Hye-Kyung Jul 07, 2025 06:09am
- With the Ministry of Food and Drug Safety planning to enforce revised GMP standards for sterile drug products reflecting PIC/S international standards in December, it plans to identify research tasks on items that require international harmonization by analyzing the regulatory status of reference countries. Ahead of its re-entry into PIC/S in 2023, the MFDS announced the “Regulations on Drug Manufacturing and Quality Control (MFDS Notice)” that contained a risk assessment-based systematic contamination management strategy and operational plan to enhance the quality assurance level of sterile drug products. Instead of implementing PIC/S-level strengthened GMP for sterile preparations in December as planned, the MFDS has decided to establish guidelines for large-volume parenteral solutions, contamination control strategies (CCS), and PUPSIT (Pre-use Post-sterilization Integrity Testing), referring to the results of the “Study on the Implementation of Sterile GMP Regulatory Harmonization” currently being conducted by the Manufacturing Quality Innovation Committee of the Korea Pharmaceutical and Bio-Pharma Manufacturers Association. Additionally, the MFDS recently announced a bid for the “Study on Implementation Strategies for Regulatory Harmonization of Manufacturing and Quality Control Based on Global GMP Trend,” aiming to establish implementation strategies for manufacturing and quality control based on an analysis of international regulatory trends, including those of advanced pharmaceutical countries and PIC/S regulations, to support the stable adoption of regulatory systems within the domestic pharmaceutical industry. The objective of the study is to establish a strategic foundation for regulatory harmonization in the pharmaceutical GMP field and expand domestic pharmaceutical exports by conducting a gap analysis based on research into international GMP regulatory trends. To this end, the regulatory status and implementation cases of advanced pharmaceutical countries and major PIC/S member countries (such as the United States, Europe, and Japan) will be studied, and gap analysis will be conducted between domestic GMP regulations and PIC/S GMP regulations to identify research tasks requiring international regulatory harmonization. In particular, the gap analysis will include the period before and after the revision of domestic GMP standards for sterile drug products, and future research tasks will include consideration of aseptic testing for large-volume parenteral solutions, the establishment of a CCS, and the application of PUPSIT. In addition, the formation of a pool of domestic and international experts in related fields, and consulting with relevant experts when necessary or holding seminars or presentations for the domestic pharmaceutical industry with invited external GMP experts as needed will also be studied. Based on the results of this study, the Ministry of Food and Drug Safety (MFDS) plans to develop draft guidelines for research topics requiring international regulatory harmonization.
- Policy
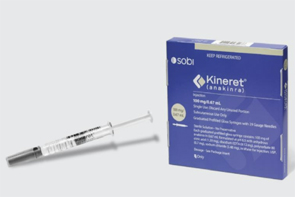
- MFDS reviews emergency import of 'anakinra'
- by Lee, Hye-Kyung Jul 07, 2025 06:08am
- Product photo of Kineret (anakinra) The Ministry of Food and Drug Safety (MFDS) is reportedly conducting a review of whether to urgently import anakinra (brand name Kineret) for the treatment of severe adverse reactions associated with CAR-T cell therapy. On June 2nd, in response to an official inquiry from specialized journalists, MFDS stated, "MFDS is currently reviewing the necessity of emergency import based on the safety, efficacy, and overseas usage status of this product and its indication." The current MFDS review is a follow-up action to the emergency import request for anakinra submitted by the Korean Society of Hematology. The Pharmaceutical Safety Bureau, which received the request, transferred the item to the MFDS Biopharmaceuticals Policy Division for an official review process. CAR-T cell therapy is an advanced, precision treatment that genetically modifies a patient's immune cells to target and attack cancer cells. It has garnered significant attention as a treatment offering different effects compared to conventional anti-cancer drugs, particularly for relapsed and refractory acute lymphoblastic leukemia, diffuse large B-cell lymphoma, and multiple myeloma. In South Korea, the treatment costs KRW 400-500 million. With national health insurance reimbursement applied, the patient's out-of-pocket burden has significantly decreased. Yet, while this high-cost therapy is supported with reimbursement, some adjuvant medications used to manage severe immune adverse reactions that can occur after treatment remain unreimbursed. 'Tocilizumab' or 'high-dose steroids' are primarily used as first-line agents. However, for patients who are refractory to these, anakinra, an Interleukin-1 (IL-1) inhibitor, serves as a crucial therapeutic alternative. However, anakinra's general use in Korea has been blocked since its withdrawal from the domestic market based on the manufacturer's decision. Anakinra is an interleukin-1 receptor antagonist manufactured by Sobi of Sweden. It's approved in countries such as the United States (FDA), Europe (EMA), and Japan (PMDA) for treating conditions including rheumatoid arthritis, CAPS (Cryopyrin-Associated Periodic Syndromes), FMF (Familial Mediterranean Fever), and Still's Disease. The 2025 European CAR T Handbook by the GoCART Coalition recommends high-dose anakinra administration for Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), and its guidelines include co-administration with corticosteroids for patients with IEC-HS (Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome). Specifically, the guidelines suggest anakinra as an essential treatment option for high-risk patients who do not respond to steroid and tocilizumab therapies. Furthermore, a study by Gazeau N et al. (Transplant Cell Ther. 2023) showed that among 43 patients who developed Grade 2 or higher complications after CAR-T therapy and were administered anakinra, the treatment-related mortality rate within 28 days was 0%, and the overall response rate was 77%. Meanwhile, in South Korea, anakinra is currently supplied only in a limited manner through the Korea Orphan & Essential Drug Center (KOEDC) as an urgently imported drug. Yet, it is not permitted for treating adverse reactions of CAR-T. Consequently, some hospitals are attempting to offer limited prescriptions through their own Institutional Review Board (IRB) approvals. However, the treatment is neither reimbursed nor guaranteed legal protection, making nationwide application impossible. The Korean Society of Hematology stated, "Currently, medical professionals are forced to off-label use anakinra, even risking illegal practice, to save patients' lives," and added, "This does not align with the fundamental principles of national public health systems and the right to life, and urgent institutional reform is desperately needed." The Korean Society of Hematology, which has submitted requests to the Ministry of Health and Welfare (MOHW) and the HIRA for several years following the reimbursement of CAR-T cell therapy, submitted a request via the 'Anti-Corruption & Civil Rights Commission (e-People)' portal in April regarding the allowance of off-label use of anakinra and institutional improvement. In response, MFDS replied that "upon request from a central administrative agency or professional organization, we can review the expansion of the reimbursement scope based on the necessity for patient treatment, availability of alternatives, and overseas approval status." In response, the Korean Society of Hematology formally submitted an emergency import request for anakinra to the MFDS Pharmaceutical Safety Bureau on May 19. The request included information such as product details, approval status in major overseas countries, usage cases in the U.S. and Europe, the necessity of its use in life-threatening situations, domestic demand prediction (less than 100 cases annually), and the lack of drug alternatives. The Korean Society of Hematology stated, "Rapid immune modulation is essential in life-threatening severe complications, and currently, there are no drugs with a similar mechanism to anakinra available in South Korea." They emphasized, "This emergency import request is an essential measure to protect patient lives and ensure access to treatment, and prompt institutional reform is required."
- Policy
- National Office of Investigation announces rebate crackdown
- by Lee, Jeong-Hwan Jul 04, 2025 06:06am
- Amidst the National Police Agency's announcement of a special crackdown on illegal drug rebates following the launch of the new administration, the Ministry of Health and Welfare is also busy assessing the situation. The MOHW plans to actively consult with the National Police Agency if it receives a specific request for cooperation to crack down on illegal rebates. On the 2nd, an official from the MOHW explained, “The issue of illegal rebates is understood to be part of a national policy agenda related to the National Police Agency's special crackdown, aimed at eradicating corruption and promoting integrity among public officials.” However, the official said that no separate request for cooperation has been made by the National Police Agency to MOHW for the special crackdown on rebates so far. The Ministry of Health and Welfare is currently maintaining its routine of monitoring cases and referring them to investigative agencies such as the police, in accordance with the guidelines for handling illegal pharmaceutical rebates. The ministry also plans to respond to future requests for cooperation. The official said, “The MOHW requests investigations by the police or the prosecution when it receives external reports or complaints related to rebates. This is in accordance with our guidelines for handling rebates. The police or the prosecution launches an investigation, then reports the results to the MOHW.” He added, “There are cases where the police request an authoritative interpretation to the MOHW when legal interpretation is necessary, such as whether there has been a violation of the Pharmaceutical Affairs Act, and some investigative agencies request the submission of expenditure reports. In response to some requests earlier this year, we provided (submitted expenditure reports) within the scope that could be disclosed.” He added, “There are no immediate plans or policies regarding the media reports, but we are cooperating with the prosecution and the police and are requesting investigations if necessary.”
- Policy
- Pressures to reform Korea's drug pricing system
- by Lee, Tak-Sun Jul 02, 2025 06:10am
- President Trump's signing of the executive order on May 12 to lower prescription drug prices in the United States to the same level as other countries has deepened the concerns of Korea’s authorities as well. This is because the U.S. Pharmaceutical Research and Manufacturers of America and others are demanding that Korea raise drug prices and improve its system. If the U.S. government directly demands improvements to Korea's drug pricing system during tariff negotiations, this may increase the new government's concerns as there will be no clear solutions to resolving the issue, On the 27th (local time), the Pharmaceutical Research and Manufacturers of America (phRMA) submitted a statement to the US Trade Representative (USTR) urging the improvement of unfair drug pricing policies by foreign governments, including South Korea, as leverage in trade negotiations. The USTR claimed that Korea imposes difficult review requirements on foreign pharmaceutical companies, delays market entry, and suppresses drug prices below fair market value. This statement gained attention because it was released after President Trump signed an executive order on May 12, known as the most-favored-nation (MFN) policy. The MFN policy aims to lower the price of prescription drugs in the US to the same level as other countries. Under the executive order, the US Secretary of Health and Human Services must establish a program that allows US patients to purchase drugs directly from pharmaceutical companies at MFN prices. In its statement, the phRMA identified South Korea, Australia, Canada, France, Germany, Italy, Japan, Spain, the United Kingdom, and the European Union as countries with unfair drug pricing policies. These countries are likely to become MFN countries, which will serve as a reference for U.S. drug price reductions. It is analyzed that the Trump administration is likely to demand that MFN countries, with which the US pharmaceutical industry is currently engaged in tariff negotiations, improve their drug pricing systems in order to push through the reduction of prescription drug prices in the US. When President Trump signed the MFN policy executive order, he instructed the USTR and the Department of Commerce to take action to prevent other countries from unfairly lowering their drug prices below market prices. The Korean government is concerned that the US MFN policy will result in ‘Korea Passing,’ where companies bypass Korea when making new drug releases and withdraw from the market. However, during Trump's first term, the US drug price reduction policy was abandoned due to opposition from US pharmaceutical companies, so the situation requires continued monitoring. However, if the US actually demands improvements to the drug pricing system during tariff negotiations, there are no clear measures that South Korea can take in response, which is expected to increase the South Korean government's concerns. The measures the South Korean government can take include significantly adjusting the ICER (Incremental Cost-Effectiveness Ratio) threshold, and expanding risk-sharing agreement programs to adjust prices. However, given the high proportion of drug expenditures in the health insurance budget, there are limits to regulatory relaxation. Flexible application of the ICER threshold or expansion of RSA programs has already been implemented last year as part of efforts to compensate for the value of innovative new drugs. If the U.S. government pushes for significant improvements to the drug pricing system under the guise of trade pressure, the new government's health authorities would have few cards to play, leaving them with even more challenges ahead. A government official stated, “We are currently monitoring and closely watching the U.S. government's implementation of the MFN policy. We are preparing for the worst-case scenario, such as the withdrawal of U.S. pharmaceutical companies' products from the market.”
- Policy
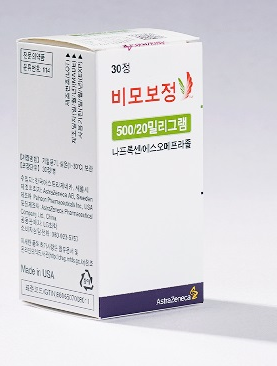
- More generic 'Vimovo' drugs with naproxen+PPI enters the mkt
- by Lee, Tak-Sun Jun 30, 2025 06:05am
- Product photo of the original drug Generic drugs containing the same active ingredients as the 'Vimovo' (naproxen+esomeprazole magnesium trihydrate), a combination of an anti-inflammatory drug and an anti-ulcer agent, are set to be released. A generic has not been approved since Chong Kun Dang's 'Naxen S Tab' was approved in 2024. Considering the characteristics of a generic containing two types of active ingredients, proving a pharmaceutical equivalence test on each active ingredient may have been challenging. Furthermore, analysis suggests new product entry to this market poses a challenge due to 'Naxozole,' a salt-changed product that was launched at a relatively lower price, with a strong presence. According to the industry on June 27, four generic Vimovo drugs, including KyungDong Pharm's 'Nasopra Tab 500/20 mg,' will be listed with reimbursement next month. KyungDong Pharm is the organizing company. The company's Nasopra Tab 500/20 mg met two types of requirements. Thus, the price was assessed at KRW 715, the same as the original Vimovo. In contrast, the drug price of the generics meeting only one requirement was set as KRW 608. These include Genuonesciences' 'Gemovo Tab 500/20 mg,' Mother's Pharmaceutical's 'Vimo M Tab. 500/20 mg,' and Dongkook Pharmaceutical's 'Exoraxen Tab 500/20 mg.' These drugs treat symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis in patients at risk of gastric or duodenal ulcers associated with NSAIDs, such as naproxen, or in those who are not adequately treated with low-dose naproxen or other NSAIDs. Joined later by four companies, products containing the same active ingredients as Vimovo have now increase from two to six. Previously, LG Chem's 'Vimovo Tab 500/20 mg' and Chong Kun Dang's 'Naxen S Tab' were the only generics available. Furthermore, Naxen S Tab has been on the market for over 10 years since its approval in 2014. Even without re-examination periods or patent barriers, generic versions have not been made available, likely due to the significant difficulty of conducting bioequivalence tests for this combination therapies. To prove bioequivalence for generic Vimovo drugs, it's necessary to compare the human absorption rates of each component, naproxen and esomeprazole. This process is complex, and achieving favorable results is challenging. Korean pharmaceutical companies have shifted their development efforts towards modified drugs that use a different salt of esomeprazole. Currently, there are five such salt-changed products available, including Hanmi Pharm's 'Naxozole Tab. 500/20 mg. Naxozole recorded KRW 25.8 billion in outpatient prescription sales last year, according to UBIST data, surpassing the original Vimovo (KRW 21.7 billion). The issue, however, is that Naxozole is relatively low-priced, at KRW 445 per tablet, which is the cheapest among competing products. Naxen S, a generic version of Vimovo, is relatively inexpensive at KRW 490 and recorded KRW 4.2 billion in outpatient prescription sales last year. In contrast, these newly reimbursed generic drugs are priced KRW 100-200 higher than Naxen S, based on the estimation criteria. Therefore, it's uncertain whether they will exhibit drug price competitiveness against Naxozole and Naxen S in the market. However, an analysis suggests that if they enter the market with high CSO (Contract Sales Organization) fees based on their higher drug prices, they could increase their market share. Amid other generic companies also preparing for market entry, attention is now focused on the market performance of the products from KyungDong Pharm and the other three companies that have recently obtained reimbursement.