- LOGIN
- MemberShip
- 2025-12-23 09:28:54
- Policy
- MFDS has completed verification of the efficacy of Retevmo
- by Lee, Tak-Sun Jan 25, 2022 05:55am
- The RET (Rearranged during transfection)) gene target anticancer drug developed by Lilly seems to be at the end of the domestic approval process. It is a drug called Retevmo (Selpercatinib), which began licensing last year, and safety and efficiency evaluation have recently been completed and is about to be approved. According to the pharmaceutical industry on the 21st, Retevmo's safety and efficiency evaluation by Lilly applied for permission, has recently ended. Analysts say that the end of safety and efficiency evaluation has further increased the possibility of product approval. In the case of new drugs, the MFDS will conduct GMP review to check safety, effectiveness, product quality, and compliance with manufacturing process standards through analysis of result data such as clinical trials to determine final product approval. The termination of safety and efficiency evaluation, which verifies drug efficacy, is interpreted as meaning that it is almost done. Retevmo is highly likely to obtain a domestic product license in that it has already received approval from the U.S. FDA based on multinational clinical trials such as data on safety and efficiency evaluation by the MFDS. At the time of FDA approval in May 2020, the drug was approved for use in adult patients with metastatic RET fusion-positive non-small cell cancer, medullary thyroid carcinoma over the age of 12, and Radioiodine-Refractory Thyroid Cancer. The RET gene is a phosphate enzyme that can cause cancer and is known to promote the proliferation of cancer cells when mutated or combined with other genes. About 2% of non-small cell carcinoma patients are found to have RET gene mutations. As RET gene mutations are also found in various carcinomas, Lilly is conducting an expanded study on Retevmo's indications. Retevmo was also designated as a rare drug in Korea by the MFDS in February last year. In addition to Retevmo, Roche's Gavreto (Pralsetinib) targeting the RET gene is also being introduced in Korea. In addition, among domestic pharmaceutical companies, HK inno.N introduced the RET target anticancer drug "VRN061782" from Voronoi in February last year and began commercialization development.
- Policy
- Xeloda in breast cancer patients is also reimbursed
- by Lee, Hye-Kyung Jan 24, 2022 05:55am
- The stage and target of administration of Roche Korea's breast cancer treatment Xeloda (Capecitabine) and Korean Pfizer's acute lymphocyte leukemia treatment Besponsa (Inotuzumab Ozogamicin) will be changed. With the establishment of a new chemotherapy benefit standard that has deleted the classification of anticancer drugs in the first and second groups, six new items, eight changes, and eight deletion items will be made in the case of urological cancer-related anticancer drug benefit standards The HIRA announced on the 18th that it will disclose the "Amendment of Announcement (proposal) according to drugs prescribed and administered to cancer patients" and conduct an opinion inquiry until the 24th. According to the revision to the announcement, on June 1, Xeloda, which "single therapy first or higher, formal chemotherapy" was changed to "second or higher" in consideration of permission and current benefit status, requested the re-establishment of the benefit standard. Xeloda is a drug licensed as a local progressive or metastatic breast cancer treatment without an anthracycline treatment plan as a result of the HIRA review that failed both chemotherapy and 'Anthracycline' drugs, and there were no authorized countries related to the first use of the administration phase. However, the textbook mentions the advantages as an oral drug and minimization of hair loss, and the NCCN guideline recommends [I,A] (consensus 71%) when prioritizing avoiding hair loss in the 'preferred category 2A' and ESMO guidelines regardless of order. The HIRA was judged as a necessary drug for treatment at the clinical site, and the administration stage and subjects of administration were changed to patients with primary metastasis and recurrent breast cancer. Besponsa is a drug licensed under the treatment of recurrent or refractory precursor B-cell acute lymphocytic leukemia (ALL) adult patients with 'the patient with relapse or refractory precursor B-cell acute lymphocytic leukemia should have failed at least one tyrosine kinase inhibitor (TKI). The NCCN guidelines recommend category 2A for Philadelphia chromosome-positive recurrence or refractory B-cell acute lymphocyte leukemia, and the HIRA also judged it as a necessary drug for treatment and provided induction therapy (500) for "philadelphia chromosome-positive recurrence or refractory precursor B-cell acute lymphocyte." Considering that Besponsa's permission from the MFDS states that the recommended administration period is 2 cycles for patients performing hematopoietic stem cell transplantation (HSCT), CR or CRi after remission induction therapy, and an additional 1 cycle will be paid 30/100. The HIRA reorganized the standards for "details on the application standards and methods of medical care benefits for drugs prescribed and administered to cancer patients" related to urinary cancer. Since last year, the HIRA has been setting up a new anti-cancer treatment benefit standard that deleted the classification of anticancer drugs in the first and second divisions after preparing a standard (draft) in related fields and collecting opinions from related societies. When the anti-cancer drug benefit standard was enacted in 2006, drugs subject to retrial, rare drugs, or abuse were classified as second-tier anticancer drugs and used within the scope of each drug's benefit standard, and clinicians were required to properly judge and administer them. A total of 21 items were revised, including 6 new items (6 therapy), 8 changed items (26 therapy), and 8 deleted items (22 therapy). According to the specific changes by cancer type, 1 kidney cancer, 1 change (10 therapy), 2 delete (10 therapy), 5 urinary epithelial cancer (6 therapy), 3 change (10 therapy), 4 delete (3 therapy), 4 prostate cancer change (6 therapy), and 2 delete (9 therapy) were carried out
- Policy
- Improve the reimb system to accommodate high-priced drugs
- by Lee, Hye-Kyung Jan 21, 2022 05:56am
- The government expressed its will to reform the reimbursement management system with the emergence of super-expensive new drugs. At the ‘Forum for the reimbursement management of high-priced pharmaceuticals,’ Yoon Seok Yang, Director of Ministry of Health and Welfare’s Division of Pharmaceutical Benefits, said, “Concern is what I first feel when a deputy director in charge reports that a new high-priced drug was released. This is why we have no choice but to agree at least in direction that we need to improve the reimbursement system, such as the risk-sharing system and the pharmacoeconomic evaluation exemption system to ensure access for the patients.” With Novartis Korea's ‘Kymriah inj (tisagenlecleucel)’ that passed deliberation by the Drug Reimbursement Evaluation Committee of the Health Insurance Review and Assessment Service marking the start, the imminent entry of ultra-high-priced one-shot treatments that cost 500 million to 2 billion won per shot including ‘Zolgensma (onasemnogene abeparvovec)’ are increasing government’s concerns. The government’s basic plan is to use the current systems in place, including the RSA and PE exemption system, to improve access to such ultra-high-priced new drugs. Yang said, “We are assessing what factors to prioritize when evaluating the cost-effectiveness of such drugs. In other words, a well-established post-management system would be most important in terms of policy.” Yang added, “As it would cost hundreds of millions of won per administration, the pharmaceutical companies would first need to present reasonable grounds for the use of such drugs, and from the perspective of managing reimbursement, we have no choice but to focus on post-management. HIRA and NHIS would have to establish a reimbursement management system.” Viewers show interest in the reimbursement listing of ultra-high-priced drugs and its post-managemen at the live YouTube forum on high-priced new drugs Ae-Ryun Kim, NHIS’s Deputy Minister of the Pharmaceutical Management Division, said “High-priced new drugs have been granted reimbursement using various systems, including the approval-reimbursement linkage system or RSA. In particular, the cost-effectiveness of high-priced drugs that were applied the PE exemption system is difficult to determine, and this is why the need for a post-management system has been continuously raised.” Kim added, “When establishing a post-management system, we need to consider the subjects, method and use and collection of data in its design. For example, HIRA believes that tighter management would be needed in the future for Kymirah, as the measure to refund a certain amount of the cost when patients do not see an effect as well as an expenditure cap was set for the use of the drug.” DREC Chair Jung Shin Lee. Emeritus Professor of Oncology at Asan Medical Center, said “Times have changed. In the past, it was all about the quality of the drug, but now discussions are focused on patient results, and end with its cost.” “Spinraza marked the start of this discussion, and more expensive drugs will continue to be introduced. As Phase III data isn’t sufficient, the only way for us to cover the drugs is by using post-management.” At the forum, criticism also arose regarding introducing ultra-high-priced drugs through the PE exemption system. Eun-Young Bae, Professor of Pharmacy at Gyeongsang National University, said “In terms of value assessment, rather than PE exemptions, work should be done to evaluate insufficient information, identify which parts are important, and collect relevant data. Also, reevaluations should be conducted for PE exemption drugs.” Patient groups on the other hand expressed that the approved drugs should be promptly listed rather than be managed for reimbursement, even if they are ultra-high-priced. Eun-Young Lee, Director of the International Alliance of Patients Organizations, said, “Safety measures and patient protection needs to be discussed thoroughly, and I know HIRA and NHIS are contemplating on how to manage reimbursement of such high-priced drugs. For high-priced drugs, prompt access to the drugs is of utmost importance, even more so than patient accessibility in general. If the patients do not get access to the drugs in time, it wouldn’t be effective. Promptness is most important.”
- Policy
- Alvogen targets the Korean market with a Spanish biosimilar
- by Lee, Tak-Sun Jan 21, 2022 05:56am
- Alvogen’s Korea is attempting to target the domestic anticancer drug market with a Spanish biosimilar. Its product is the third Avastin (bevacizumab) biosimilar to enter the market after Samsung Bioepis and Pfizer. On whether the new biosimilar will become a new sensation among latecomers in the market and is gaining attention. On the 19th, the Ministry of Food and Drug Safety approved Alvogen Korea’s ‘Arimsis inj.’ a monoclonal antibody treatment for cancer that contains the same ingredient as Roche Korea’s ‘Avastin inj.’ A clinical trial on 625 healthy male adults or patients with advanced or metastatic non-squamous non-small-cell lung cancer was conducted to assess the drug’s equivalence in pharmacodynamics and efficacy to its comparator. The drug's pharmacodynamic equivalence was demonstrated in 114 healthy male adult participants who received 'Arimsis' and its comparator Avastin. Also, the efficacy of the drug was verified through a study on 511 patients with advanced or metastatic non-squamous non-small-cell lung cancer. Results showed that the objective response rate (ORR) of the patients was statistically equivalent to that of its comparator. Until now, only a few biotech companies attempted to develop biosimilars due to the large-scale facilities required. Through active investment in such facilities, the Korean companies Celltrion and Samsung Bioepis have been dominating the global biosimilar market. However, global pharmaceutical companies have also recently joined in the competition. Amgen&Allergan and Pfizer received US FDA's approval for their Avastin biosimilars. Also, Samsung Bioepis and Celltrion are attempting to receive FDA approval for their Avastin biosimilars. In Korea, Samsung Bioepis’ ‘Onbevzi,’ and Pfizer’s ‘Zirabev’ received approval one after another and are soon to compete in the domestic market. Alvogen’s biosimilar ‘Arimsis’ was developed by a less well-known Spanish pharmaceutical company, mAbxience Research. mAbxience is a biotech company that specializes in the development of biosimilars. The company, which was established in 2020, had been developing biosimilars of Mabthera (rituximab) and Avastin (bevacizumab). Its Avastin biosimilar was first approved in Latin America in 2016, then approved by the European Commission last year. The company has applied for the drug’s approval to the US FDA in June last year. Alvogen succeeded in becoming the third Avastin biosimilar to be approved in Korea using mAbxience’s product that had already been approved overseas. In particular, this product has the same indication as Avastin. Avastin and Arimsis are indicated for ▲metastatic colorectal cancer ▲metastatic breast cancer ▲non-small cell lung cancer ▲ advanced or metastatic renal cell carcinoma ▲glioblastoma ▲epithelial ovarian cancer, fallopian tube, or primary peritoneal cancer, and ▲cervical cancer. On the other hand, the indication for Samsung Bioepis’s ‘Onbevzi inj.’ excludes second-line treatment of epithelial ovarian cancer, fallopian tube, or primary peritoneal cancer to evade Avastin’s use patent. Alvogen first filed a trial to invalidate the use patent of Avastin in August. Samsung Bioepis also joined in the patent challenge after Alvogen filed the claims.
- Policy
- Pt management is required to provide high priced 1 shot tx
- by Lee, Hye-Kyung Jan 21, 2022 05:56am
- Starting with Kymriah of Novartis Korea, which recently passed the HIRA Drug Reimbursement Evaluation Committee, the search for management measures for ultra-high-priced one-shot treatments has begun with Zolgensma, which is expected to enter Korea. In the end, the government's plan to manage the benefits of expensive drugs is a contract that uses treatments as DLBCL, but turns the benefits for patients who do not work into sharing by pharmaceutical companies. Kymriah, which passed the Drug Reimbursement Evaluation Committee on the 13th, was also recognized for his eligibility, but conditions for applying DLBCL and Expenditure Cap have been attached, and all expensive drugs are likely to follow the contract in the future. Byun Ji-hye, a deputy researcher at HIRA, announced domestic benefit plans such as expensive drugs using actual clinical grounds at the "Expensive Drug Benefit Management Forum" held on the 19th, saying, "We need to think about how to manage patients who have administered but are ineffective. I think pharmaceutical companies should also bear the list." In the case of the SMA treatment Spinraza (Nusinersen), which is managed by the pre-approval system, Spinraza has been administered for two years, but there are several cases where the exercise function evaluation score continues to be "0". She said, "In Korea, there are no specific standards for maintaining and improving exercise functions." She said, "We believe that performance-based benefit management is necessary by setting the minimum score for improving exercise function, which is clinically meaningful, and considering clinical improvement scores." Spinraza is an ultra-high-priced new drug with an insurance cap of 92.35 million won per bottle of 5ml, and nursing institutions that want to take it must apply for pre-approval and submit monitoring reports every four months after approval of benefit. When drug price negotiations with the NHIS are completed soon, Kymriah, which is scheduled to be registered, and Zolgensma, which is expected to enter Korea, will have to use the pre-approval system like Spinraza. The HIRA's high-drug benefit management plan also suggested approval and monitoring of patients subject to administration using the pre-approval system, financial management using risk-sharing systems (RSA), benefit standards based on revaluation results, and domestic clinical guidelines. Deputy researcher Byun said, "Expensive drugs should be administered to optimal target patients for efficient use of limited resources. Monitoring and re-evaluation should be conducted through patient-level data collection by establishing a disease-level registry." It also came up with financial management plans such as refunding patients' events during the monitoring period of pharmaceutical companies, adjusting the refund ratio reflecting the evaluation results after the end of the contract period, and setting the total amount considering the financial impact. Deputy researcher Byun emphasized, "benefit standards and domestic clinical guidelines are also needed, such as collecting essential clinical information for screening and evaluation and analyzing it with related clinical societies when re-evaluating in connection with health care big data such as billing data."
- Policy
- Dong-A ST enters Phase III trial for its new OAB treatment
- by Lee, Tak-Sun Jan 20, 2022 05:55am
- Dong-A STNews of new drugs being developed by local companies are coming in one after another from early on this year. Dong-A ST, which had developed three new drugs including the erectile dysfunction treatment ‘Zydena (udenafil),’ antibiotic ‘Sivestro (tedizolid phosphate),’ and antidiabetic ‘Suganon (evogliptin),’ is also receiving attention with its new drug candidate entering final stages of commercialization. The Ministry of Food and Drug Safety approved the Phase III study protocol for Dong-A ST’s 'DA-8010,’ a new drug candidate in development for the treatment of overactive bladder syndrome. The Phase III trial will be conducted to test the efficacy and safety of DA-8010 in 595 patients with overactive bladder in Korea. The trial will be conducted at the Sinchon Severance Hospital. DA-8010 is a new antimuscarinic being developed by Dong-A ST. Antimuscarinics inhibit involuntary contraction of the bladder and reduce urinary urgency to increase bladder capacity by primarily acting in the urinary storage phase where parasympathetic nerves are not activated. Astellas ‘VESIcare (solifenacin)’ is a representative antimuscarinic drug, However, antimuscarinic drugs as such are associated with adverse events such as dry mouth and constipation. DA-8010 is expected to improve such side effects and have a more superior effect over existing products. Dong-A ST had started the development of the drug in 2010 last year and is now awaiting commercialization. In addition to Korea, the company has also completed a Phase I trial for the drug in Europe. Dong-A ST, which is famous for its energy drink ‘Bacchus,’ has been leading the R&D of new drugs in the domestic industry, succeeding in developing 3 new drugs. In particular, its ‘Zydena (erectile dysfunction)’ that was approved in 2005 and ‘Suganon (diabetes treatment)’ in 2015 both made great success in the market and were noted for their marketability as well. ‘4 new homegrown drugs’ approved last year… Daewoong and SK Bioscience expected to again receive approval for new drugs this year Yuhan Corp’s new NSCLC treatment that was approved in January last yearLast year, Korea celebrated the highest number of approvals of homegrown new drugs. Starting with Yuhan Corp’s anticancer drug ‘Leclaza’ in January, Celltrion’s COVID-19 treatment ‘Regkirona’ in February, Hanmi Pharmaceutical’s neutropenia treatment ‘Rolontis’ in March, and Daewoong Pharmaceutical's ‘Fexclu’ was approved in December last year. The companies are developing their drugs abroad as well to target the global market. In the case of Rolontis, the industry believes the drug may be approved by the US FDA within the year. Daewoong Pharmaceutical, which received approval for Fexclu tab in the end of last year, is also planning to introduce a new drug this year. The company plans to complete Phase III trials for the SGLT-2 inhibitor it is developing for the treatment of type 2 diabetes, 'DWP16001,' as a monotherapy and combination therapy. The COVID-19 vaccine candidate ‘GBP510’ that is being developed by SK Bioscience also completed patient enrollment for the Phase III trial by registering 4,000 patients and started the efficacy analysis. GBP5010 is expected to be commercialized in the first half of this year.
- Policy
- SK Bioscience has completed recruiting phase 3 participants
- by Kim, Jung-Ju Jan 20, 2022 05:55am
- The COVID-19 vaccine GBP510, which is being developed by Korean companies, is undergoing phase 3 global clinical trials. The recruitment of 4,000 people from six countries has been completed. Today (18th), the government held the 26th meeting of the pan-government TF to support clinical trials of COVID-19 vaccines and treatments to discuss the progress of the development of COVID-19 vaccines in Korea. The meeting was attended by Ryu Geun-hyuk, the second vice minister of the MOHW, related ministries, and private experts, and SK Bioscience President Ahn Jae-yong announced the progress of clinical trials for the COVID-19 vaccine currently under development. In Korea, eight companies are currently conducting clinical trials for the COVID-19 vaccine. Participating companies are SK Bioscience, EuBiologics, Geneone, Genexine, Quratis, HK inno.N, Cellid, Aijin. Among them, SK Bioscience's GBP510 is being developed the fastest with its entry into phase 3 clinical trials as of August 10 last year. Phase 3 clinical trials of GBP510 have recruited clinical participants from five foreign countries, including Korea, Thailand, the Philippines, Vietnam, Ukraine, and New Zealand, with a total of 3,990 subjects in five months since it began administration on August 30 last year. SK Bioscience announced that it plans to complete the development of vaccines in the first half of this year through rapid sample analysis and data acquisition for vaccines under development in the future. When the development of GBP510 is completed with CEPI support, it will be supplied to countries around the world through the COVAX Facility. Meanwhile, the government is also conducting a pre-purchase process for 10 million doses of domestic vaccines under development by SK Bioscience. Until now, the government has supported the recruitment of clinical participants, supported rapid clinical proceedings such as clinical permits through overseas missions in clinical countries, and supported rapid sample analysis led by the government for vaccine efficacy analysis through Korea National Institute of Health and the International Vaccine Institute. As of the 17th, a total of 2,163 cases have been received, and 1,764 cases have been analyzed, and the sample analysis is supported to proceed quickly as soon as the sample is received. In addition, a cross- and additional vaccination clinical trial under the supervision of KDCA is underway for the safe vaccination of the people after the development of domestic vaccines. Second Vice Minister Ryu Geun-hyuk said, "Even when it is difficult to recruit clinical participants due to COVID-19 vaccination, we were able to complete the recruitment of clinical trial participants for domestic vaccine development smoothly thanks to the public's high interest and active cooperation. After that, we will focus on support at the pan-government level so that sample analysis, approval, examination, and commercialization can proceed quickly. We will support the development of various domestic vaccines and treatments, including SK Bioscience, until the end."
- Policy
- Additional Paxlovid for 10,000 people will be introduced
- by Kang, Shin-Kook Jan 20, 2022 05:54am
- Prime Minister Kim Bu-gyeom visited the Residential Treatment CenterPrime Minister Kim Bu-gyeom announced that he would introduce an additional 10,000 servings at the end of this month as patients' condition improved after taking the COVID-19 treatment. Prime Minister Kim posted a message on his SNS on the 17th and said, "The prescription and administration of COVID-19 treatment began last week." He said, "I visited the residential treatment center in Jung-gu, Seoul a while ago and looked at the status of medication for eating treatments. "I met the elderly who were taking the medicine on the screen and asked how they were doing over the phone, and I heard that the elderly were taking the medicine well and are currently improving well without any adverse reactions." He said, "In addition to the elderly I called today, it is very fortunate that patients who were prescribed during home treatment are being treated without any major adverse reactions. There are drugs that patient shouldn't take together, so patient needs to be careful with prescriptions, and speed is important because he/she has to take the medication within five days of symptoms." He said, "Looking at the scene today, if the prescribed person is taking a medicine that should not be taken together, the notification function is immediately activated on the system. "We were well prepared for quick and safe administration through the world-class drug safety use service (DUR)." Prime Minister Kim said, "The introduction of treatments is also proceeding without much difficulty." He said, "Last week, Doses for 21,000 people were introduced, and an additional doses for 10,000 people will be arrived at the end of this month. If active prescriptions begin, the severity rate and mortality rate will be lowered through the treatment taken, which will be a good response to Omicron mutations, he predicted. In addition, he said, "The government will thoroughly manage the monitoring of adverse reactions of treatments to be taken and actively respond to side effects."
- Policy
- Bill for Rotarix·RotaTeq to be covered by NIP
- by Lee, Jeong-Hwan Jan 19, 2022 06:06am
- A bill to promote the health of infants and young children while reducing consumer burden by including rotavirus vaccines in the National Immunization Program (NIP) is being promoted. If passed, the bill will turn GSK’s Rotarix and MSD’s RotaTeq that are already being used in Korea into free national vaccinations. Since the WHO recommends mandatory vaccination of the rotavirus vaccine and the nature of the virus that is highly infectious among infants and young children, attention is focused on the legislative direction of the bill. On the 18th, NA member Bae Hyun-jin of the People Power Party announced that she had submitted a bill as representative for the partial amendment of the ‘Infectious Disease Control And Prevention Act’ to incorporate such changes. The incidence of rotavirus infection is very high among children aged under 5 to the extent that most of these infants are infected at least once with the virus. NA member Bae Hyun-jin expressed concerns that some parents give up rotavirus vaccinations due to the high cost even though rotavirus vaccinations are recommended for all newborns after 6 weeks of age. According to the KDCA’s Immunization Registry System, 7.9%, 21,728 of the 274,221 newborns eligible for vaccinations did not receive rotavirus vaccines in 2020. Statistics over the past 5 years show that 14.8% of the infants and young children eligible for vaccinations – 222,565 – did not receive rotavirus vaccines. Rotavirus vaccines are relatively high-priced, costing around ₩200,000-₩300,000. The two rotavirus vaccines currently sold in the market are GSK’s Rotarix and MSD’s RotaTeq. Rotarix is given in 2 doses, RotaTeq in 3. The two vaccines cost ₩70,000-₩100,000 and ₩100,000-₩130,000 per shot, respectively. In total, Rotarix costs around ₩200,000-₩260,000 and RotaTeq ₩210,000-₩300,000 to get fully vaccinated. The issue regarding the price of rotavirus vaccines has risen several times intermittently. GSK and MSD had raised the price of Rotarix and RotaTeq by 12% and 17% respectively, increasing the burden borne by consumers. Rotavirus vaccines are considered essential for newborns, and therefore its consumers are highly sensitive to their change in price. As a result, some local governments have implemented policies to provide free rotavirus vaccines for infants. Bae believes that the government should pay for the cost of the rotavirus vaccine inoculations to prevent enteritis in infants and young children, contribute to promoting public health, and reduce the burden borne by the consumers. Therefore, the bill contains a plan to include rotavirus infection as a disease eligible for regular vaccinations. Bae said, “The reality is that the need for rotavirus vaccination has been discussed for a long time but was excluded from support due to its high cost. However, no infants nor young children should be left out, unable to receive essential vaccinations due to financial reasons.”
- Policy
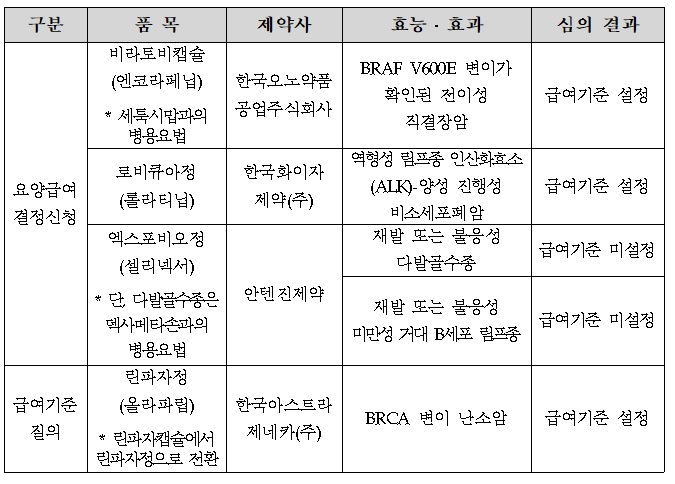
- The benefit standard for Braftovi & Lorviqua was set
- by Lee, Hye-Kyung Jan 19, 2022 06:06am
- At the first Severe (Cancer) Disease Review Committee held this year, the benefit standards for Braftovi, a direct bowel cancer treatment of Ono Pharmaceutical Korea, and Pfizer's Lorviqua, were set. The HIRA (Director Kim Sun-min) today (12th) released the "Result of Review on benefit Standards for Drugs for Cancer Patients" reviewed by the 1st Cancer Disease Review Committee in 2022. Today's cancer screening was newly participated by members of the 9th committee formed in November last year, and Braftovi and Lorviqua, who applied for medical care benefit decisions, set benefit standards, and Antigene's Xpovio did not set standards. Braftovi, which standard was set, was approved by the MFDS on August 19 last year for the use of combination therapy with Erbitux in adult patients with direct bowel cancer who had previous treatment experience and confirmed BRAF V600E mutation. Since Lorviqua was designated as a rare drug in March 2020, it is approved in Korea in July 2021 for use in cases treated with Xalkori and at least one other ALK inhibitor, or with a primary ALK inhibitor as a monotherapy for the treatment of adult patients with ALK-positive progressive non-small cell lung cancer. Xpovio has not established a benefit standard for both permits, such as recurrent or refractory multiple myeloma and recurrent or refractory giant B cell lymphoma. The standard was set for Lynparza tab's standard. Meanwhile, in accordance with Articles 5 and 5-2 of the National Health Insurance Medical Care Benefit Standards, the HIRA may publicly announce drugs prescribed and administered to severely ill patients after deliberation by the Severe Disease Review Committee. The benefit criteria for the drug may be set differently according to clinical literature, domestic and foreign guidelines, and expert opinions within the scope of the efficacy and effectiveness of the MFDS' permission, and the benefit criteria may change during the follow-up procedure.