- LOGIN
- MemberShip
- 2025-12-19 17:27:19
- Policy
- Measures to ban imports of psychotic dietary supplements
- by Lee, Hye-Kyung Jul 28, 2025 06:07am
- The Ministry of Food and Drug Safety (MFDS; Minister, Yu-Kyoung Oh) announced on July 25 that the Ministry is designating '7-Hydroxymitragynine,' an ingredient that is narcotic (antipsychotic), as a prohibited material and ingredient for import into South Korea. This measure was implemented to ban overseas direct purchase of dietary supplements, gummies, or drink mixes containing '7-Hydroxymitragynine.' The newly designated 7-hydroxymitragynine is an ingredient classified as a psychotropic substance under 'The Narcotics Control Act.' It is an alkaloid component present in small amounts in the Southeast Asian plant Mitragyna speciosa (scientific name), commonly known as Kratom. It is known to pose a serious risk to human health if misused or abused. The Ministry of Food and Drug Safety is strengthening the safety management of overseas direct-purchase foods. To this end, it designates ingredients and raw materials (such as narcotics, medicinal and traditional Korean medicine ingredients) in overseas direct-purchase foods that pose a risk to public health and need to be blocked from domestic entry, as raw materials/ingredients subject to domestic import blockage. Furthermore, considering that consumers find it difficult to identify harmful ingredients/raw materials, MFDS also provides an easily understandable list of products containing harmful ingredients (3,800 items). Therefore, it is vital for consumers first to check the 'Overseas Direct Purchase Foods' section on the 'FoodSafetyKorea' website before purchasing overseas direct-purchase foods. MFDS stated, "We plan to continue providing consumers with information on precautions and harmful substances when purchasing overseas direct purchase foods."
- Policy
- Daewoong, Kolon enter Ofev generic market at the same price
- by Lee, Tak-Sun Jul 25, 2025 06:09am
- Daewoong Pharmaceutical and Kolon Pharma will additionally enter the generic market for “Ofev Soft Cap (Nintedanib Esylate),” a treatment for chronic fibrotic interstitial lung disease. Competition is expected to intensify in the market with two more generic versions entering the market following Yungjin and Ildong Pharmaceutical’s entry with their respective generic versions in July. Except for the Ildong Pharmaceutical product, the prices of the generic drugs were all set the same. According to industry sources on the 24th, Daewoong Pharmaceutical's Ofild Tab 100 mg and 150 mg and Kolon Pharma’s Effidanib Tab 100 mg and 150 mg will be reimbursed from August in Korea. These generic versions contain the same active ingredient as Ofev Soft Cap (nintedanib esilate), but differ in formulation — the original is a soft capsule, while the generics are in tablet form. Ofev was listed for reimbursement in May as a treatment for chronic fibrotic interstitial lung disease. It took nine years for the company to obtain reimbursement after receiving marketing authorization from the MFDS. There are currently no alternative therapeutic options recognized for chronic fibrosing interstitial lung diseases in Korea. However, the reimbursement came too late, as it came even after the drug’s substance patent expiry on January 25, 2025. Two months after Ofev was listed for reimbursement, its generic versions started to appear on the market. Youngjin Pharmaceutical's Nintebro Tab 100 mg and 150 mg, and Ildong Pharmaceutical's Cuninta Tab 150 mg were the first generics to be listed for reimbursement. A month later, Daewoong Pharmaceutical and Kolon Pharma also entered the generic market. Although all generic drugs are designated as orphan drugs and could have been priced the same as the original Ofev, all the companies decided to lower their prices. Coincidentally, except for Ildong Pharmaceutical, all other pharmaceutical companies set the same prices for their respective products. The companies’ 100mg dose version is priced at KRW 9,000, and the 150mg at KRW 15,000. Ildong Pharmaceutical's Cuninta Tab 150 mg is the cheapest at KRW 13,500. The original Ofev soft Cap cost KRW 29,600 for the 100 mg dose and KRW 26,220 for the 150 mg dose, which is about twice the price of the generic drugs. The generic drugmakers sought to quickly enter the market with low prices. In particular, with only a 2-3 month difference in release dates compared to the original, industry observers believe that the generic companies stand a chance of gaining an equal market share. The estimated annual national health insurance financial expenditure for listing Ofev was KRW 6.3 billion. This calculation is based on the assumption that approximately 329 patients will be prescribed 2 capsules per day. Although the claim amount is not large, at less than KRW 10 billion, pharmaceutical companies have entered competition because the market is essentially untapped. Latecomers are particularly expecting synergistic effects with pirfenidone, which is already used for idiopathic pulmonary fibrosis. This is because nintedanib is already being used without reimbursement for idiopathic pulmonary fibrosis, and the hospitals treating these patients tend to be the same institutions that prescribe pirfenidone. In a market where the original and generic drugs are launched almost simultaneously, all eyes are on who will emerge as the ultimate winner.
- Policy
- Reimb criteria established for Roche's DLBCL drug 'Polivy'
- by Lee, Tak-Sun Jul 25, 2025 06:08am
- The reimbursement criteria for Roche Korea's Polivy (polatuzumab vedotin), a treatment for relapsed or refractory diffuse large B-cell lymphoma (DLBCL), have been successfully established by the Health Insurance Review & Assessment Service's (HIRA) Cancer Disease Review Committee (CDRC). This drug, which is garnering attention as the first DLBCL first-line treatment in 20 years, passed the CDRC on its third attempt. HIRA announced that reimbursement criteria were established for drugs, including Polivy, at the 6th CDRC meeting held on July 23, 2025. For Polivy, the reimbursement criteria were set for "combination therapy with rituximab, cyclophosphamide, doxorubicin, and prednisone/prednisolone (R-CHP) in adult patients with previously untreated diffuse large B-Cell lymphoma (DLBCL)." Meanwhile, the reimbursement criteria have not been established for Polivy in combination with bendamustine and rituximab in adult patients with relapsed or refractory DLBCL who are not eligible for hematopoietic stem cell transplantation and have failed one or more prior therapies. Along with Polivy, reimbursement criteria were established for the new drug Darzalex SC (daratumumab) for "combination therapy with bortezomib, cyclophosphamide, and dexamethasone in newly diagnosed light chain (AL) amyloidosis patients." Reimbursement criteria have been established for Polivy (polatuzumab vedotin) and Darzalex SC (daratumumab). Expanded reimbursement criteria were approved for Neulasta Prefilled Syringe, Tecentriq, and Blincyto. Drugs that failed to receive the reimbursement criteria include Verzenio (abemaciclib). Expanded reimbursement criteria were approved for Neulasta Prefilled Syringe, Tecentriq, and Blincyto. For Neulasta, the reimbursement criteria were set for "reduction in the incidence of febrile neutropenia and the duration of neutropenia in patients receiving cytotoxic chemotherapy for malignant neoplasms." For Tecentriq, reimbursement criteria were established for "post-operative adjuvant therapy after resection and platinum-based chemotherapy in patients with Stage II-IIIA non-small cell lung cancer (NSCLC) where PD-L1 is expressed in ≥ 50% of tumor cells (TC)." For Blincyto, reimbursement criteria were set for "consolidation therapy for Philadelphia chromosome-negative precursor B-cell acute lymphoblastic leukemia (ALL) in adults and children." The anticancer drugs for which reimbursement criteria have now been established will undergo final review for reimbursement coverage through negotiation with HIRA's Drug Reimbursement Evaluation Committee (DREC) and the National Health Insurance Service.
- Policy
- Eun-Kyung Jeong appointed as new MOHW Minister
- by Lee, Jeong-Hwan Jul 24, 2025 11:38pm
- With the appointment of Eun-kyung Jeong (60, Seoul National University College of Medicine) as Minister of Health and Welfare, efforts to institutionalize and legislate telemedicine, as well as to establish a prescription drug delivery system, are expected to gain momentum. The government will begin establishing specific measures to stabilize the supply of pharmaceuticals, including promoting generic prescriptions and limited introduction of International Nonproprietary Name (INN) prescriptions. In light of the government’s push to strengthen health insurance coverage, the possibility of reforming the pricing system for generic drugs is also increasing. For this, the new minister will be tasked with addressing demands from the pharmaceutical industry. On the 22nd, Minister Jung highlighted the key healthcare and pharmaceutical policies she expects to pursue during her inaugural speech. Rapid rollout of telemedicine and prescription drug delivery policies Minister Jung plans to establish a legal framework for telemedicine as a supplementary tool to in-person care, aiming to enhance the stability of the system. She plans to actively participate in the enactment of three bills on revising the Medical Service Act currently pending in the National Assembly that focus on institutionalizing telemedicine. In the process, a public electronic prescription system for telemedicine and a prescription drug delivery system will likely be established. Minister Jung has repeatedly emphasized her commitment to legislating telemedicine in a way that prevents intermediary platforms from dominating medical institutions and pharmacies, thereby avoiding disruption to the healthcare delivery system and the pharmacy ecosystem. Accordingly, detailed provisions for the regulation and oversight of telemedicine intermediary platforms are expected to be carefully incorporated during the legislative process. “In light of the need to ensure medical safety and convenience for the public, as well as to improve healthcare accessibility for vulnerable populations, it is essential to establish a legal framework for telemedicine and intermediary platform businesses to enable stable operation. We will gather opinions from various stakeholders, including the medical and pharmaceutical community, patient groups, private companies, and experts, to systematically establish a plan from the outset for the construction and operation of a public electronic prescription transmission system.” She added, “Telemedicine should be institutionalized in a way that ensures the safety of medical treatment and improves the quality of primary care, rather than expanding the profits of platforms. I hope that appropriate regulatory measures will be discussed in the National Assembly to address concerns that platforms may effectively demonize medical institutions and pharmacies or promote profit maximization.” Regarding the institutionalization of a drug delivery system to improve the effectiveness of telemedicine, Jung said, “We agree on the need to establish a drug delivery system,” but added, “However, we must also consider measures to address concerns about the pharmacy dependence within telemedicine platforms, market concentration of large pharmacies, and the collapse of the local pharmacy system.” Regarding the major point of contention, the scope of initial and follow-up telemedicine consultations, Minister Jung responded, “We will discuss the matter after evaluating the pilot program and comprehensively considering the opinions of experts, the medical community, and patients, but we will follow medical judgments and standards rather than administrative criteria.” Generic drug price cut is likely With Minister Jung’s appointment, there is a greater possibility that drug price reevaluation, which will reduce the prices of generic drugs that account for a large proportion of domestic pharmaceutical companies, will be activated. Although it is challenging to compare drug prices in real terms due to differences in economic scales, drug pricing systems, and health insurance systems across countries, Minister Jung’s view is that the price of generic drugs in Korea is higher compared to other major countries (A8). To provide optimal drug reimbursement within the limited health insurance budget, Minister Jung had previously stressed the need to manage drug prices at an appropriate level. Minister Jung’s solution involves reforming the drug pricing reimbursement system to ensure that profits from generic drug sales can be reinvested into new drug development, while also preventing excessive competition from a flood of generics with identical ingredients and manufacturing processes produced through contract manufacturing. In particular, regarding the post-market management of drug prices, such as regular re-evaluation of reimbursement prices and benefit criteria, Minister Jung reaffirmed plans to revise the pricing policy for generics, stating “I believe there is room for stronger oversight in the pricing system, particularly concerning generic drugs.” Improvement of measures for drug with unstable supply and review of INN prescriptions In order to resolve the issue of unstable drug supply, there is a possibility that a new supply response system with the nature of an intergovernmental consultative body will be established, or that relevant legislation such as the revision of the Pharmaceutical Affairs Act will be enacted. This is because Minister Jeong agreed on the need to break away from the current limitations of seeking solutions to the unstable drug supply situation only around essential medicines. During the hearing, Minister Jung stated, “Supply instability issues occur regardless of whether they are classified as essential medications, so the current support system that focuses on essential medications under the Pharmaceutical Affairs Act has limitations in addressing such issues. Therefore, it is necessary to revise the support system under the Pharmaceutical Affairs Act to enable policy intervention across all aspects of medicines with unstable supply, including the establishment of new support grounds and governance reforms.” In particular, unlike national essential medicines, there are no legal grounds for defining medicines with unstable supply, so the Minister agreed on the need to establish a separate definition. In addition, Minister Jung is likely to take proactive measures beyond diagnosing causes and providing customized support through public-private consultations on medicines with unstable supply, such as promoting substitute prescriptions and limited introduction of International Nonproprietary Name (INN) prescriptions. Minister Jung explained, “To enable flexible responses to drug supply instability situations, we have revised the Enforcement Decree of the Pharmaceutical Affairs Act to simplify the post-notification procedure for substitute prescriptions. We are currently constructing a support information system for post-notification of substitute prescriptions in line with the implementation date of the revised Enforcement Decree.” She further elaborated, “Additionally, to fulfill the new government's pledge to ensure the stable supply of essential medicines, we are considering the introduction of INN prescriptions for essential medicines with unstable supply.”
- Policy
- Jeong Eun-kyung views "orphan disease fund establishment"
- by Lee, Jeong-Hwan Jul 24, 2025 11:38pm
- Jung Eun-kyung, the candidate for Minister of Health and Welfare (MOHW), has expressed skepticism regarding the establishment of a separate fund to improve the National Health Insurance reimbursement rate for treatments for expensive, rare (orphan), and severe diseases. The rationale is that if the required financial scale for reimbursing high-cost drugs exceeds the fund's size, flexible fund operation becomes difficult. Jung stated her view that continuously expanding the scope of reimbursement should take precedence over establishing a separate fund. On July 17, Jung provided this response to a written inquiry from Democratic Party of Korea Representative Jeon Jin-suk during the parliamentary confirmation hearing. Representative Jeon had asked for Jung's stance on strengthening coverage for orphan and severe disease treatments by creating a separate dedicated fund to improve the National Health Insurance reimbursement listing system. Jung acknowledged that establishing and operating a separate fund for patients with orphan and severe diseases would have the advantage of allowing independent financial management. However, she did not prioritize improving the reimbursement rate. Jung stated, "If the required financial resources for high-cost treatments exceed the fund's scale, there is a concern that flexible operation would become difficult." She emphasized, "Therefore, continuously expanding the scope of reimbursement is primarily needed rather than establishing a separate fund." Jung added, "Currently, various support measures are in operation, including 'special calculation and improvement of standards' to alleviate out-of-pocket burdens and catastrophic medical expense support," and added, "We will strive to strengthen coverage for rare and severe disease patients." Regarding the introduction of an indication-specific drug pricing system for recently developed immunotherapies for cancer that have single components but multiple indications, Jung provided a principled answer that she would review it. Jung said, "It is necessary to review various systems to strengthen patient access to recently developed new drugs." However, she added, "We will review it comprehensively, considering its suitability for operation within the National Health Insurance system in Korea, the benefits of introducing the system, and its financial and social impact."
- Policy
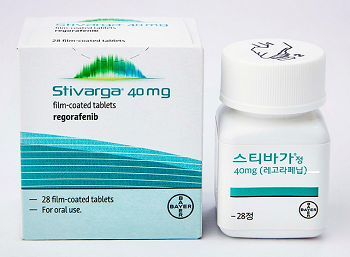
- Release of Boryung’s generic version of Stivarga imminent
- by Lee, Tak-Sun Jul 24, 2025 06:07am
- The price of the liver cancer drug Stivarga Tab 40mg (regorafenib, Bayer) will be adjusted following the termination of the risk-sharing agreement due to the anticipated listing of its generic versions. Among the many companies, Boryung is expected to be the first company to launch a generic version of Stivarga in Korea. According to industry sources on the 23rd, the maximum price of Stivarga Tab 40mg will be adjusted from KRW 28,110 to KRW 22,450 from August. This is a measure following the termination of the risk-sharing agreement. Stivarga entered into a refund-type risk-sharing agreement when it was listed for reimbursement in June 2016 as a treatment for gastrointestinal stromal tumor (GIST). In addition, from May 2018, a refund-type RSA has been additionally applied to Stivarga as a second-line treatment for hepatocellular carcinoma. Last year, the company also signed a third RSA renewal contract with the Health Insurance Review and Assessment Service. The current insurance price cap is set at KRW 28,110, different from the actual price. However, the insurance price cap of KRW 22,450, to which the price will be adjusted after the termination of the RSA deal, is the actual price. The RSA has been in effect for 9 years, but a variable–entry of generic versions has emerged. In November last year, Boryung Pharmacuetical received approval from the MFDS for Regoranib Tab 40 mg, a generic version with the same active ingredient as Stivarga. Regoranib demonstrated bioequivalence to Stivarga through a bioequivalence test. This drug is expected to be released on the market after August 29, when Stivarga's patent expires. The industry expects it to be distributed on the market from September. The termination of the RSA for Stivarga was decided at the Drug Reimbursement Evaluation Committee (DREC) meeting held in April. The committee concluded that, considering Stivarga 40mg is expected to remain reimbursable even after the marketing of an equivalent generic formulation, the product qualifies for early termination of the RSA and that termination negotiations are required. Since then, the company completed negotiations to terminate the risk-sharing agreement with NHIS, and the insurance price ceiling amount was adjusted. Last year, Stivarga’s outpatient prescription sales amounted to KRW 7.7 billion, a decrease of 16.8% compared to the previous year. Since February, Chong Kun Dang has been exclusively distributing the drug in Korea under a contract with Bayer.
- Policy
- Ruling party introduces another bill to reimburse abortion
- by Lee, Jeong-Hwan Jul 24, 2025 06:07am
- Following Representative In-soon Nam of the Democratic Party of Korea, Representative Sujin Lee of the same party submitted a bill on the 23rd to legally allow artificial termination of pregnancy through drugs such as Mifegyne and to apply health insurance reimbursement to surgical and pharmaceutical abortion procedures. The partial amendment to the Mother and Child Health Act proposed by Rep Sujin Lee allows artificial termination of pregnancy with the consent of the pregnant woman and provides insurance reimbursement for relevant procedures. Also, the bill will revise the term "artificial abortion surgery" to "artificial pregnancy termination," and the Minister of Health and Welfare will be granted legal authority to establish and operate pregnancy and childbirth support centers. These centers will handle services such as emergency hotlines and online counseling for pregnancy and childbirth-related support. Local government heads will be required to establish comprehensive counseling centers within public health centers, or the Minister of Health and Welfare may designate such institutions to provide information on pregnancy and childbirth, and to conduct counseling related to both maintenance and termination of pregnancy. Under the proposed legislation, the head of the comprehensive counseling center will be required to promptly issue a certificate confirming the counseling session when requested by a woman who has received counseling on maintaining or terminating a pregnancy. Rep Lee explained, “Although the Constitutional Court has ruled that the crimes of self-induced abortion and physician-assisted abortion are unconstitutional, relevant laws have not yet been revised, resulting in a legislative gap. This legislation aims to allow the use of medication for pregnancy termination, permit artificial pregnancy termination without restrictions, and ensure that women can make their own decisions through adequate information and support.” Abortion pills are available overseas under the name “Mifegyne.” In South Korea, Hyundai Pharm has reportedly signed a licensing agreement and exclusive supply contract with British pharmaceutical company LinePharma International for the domestic distribution of the drug under the brand name “Mifegymiso.”
- Policy
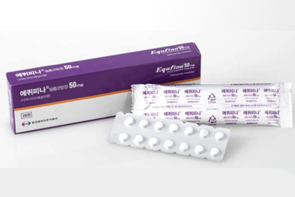
- Supply shortage of Parkinson’s drug Equfina is expected
- by Lee, Hye-Kyung Jul 23, 2025 06:08am
- Eisai Korea's Parkinson's disease treatment ‘Equfina Film-Coated Tab 50mg (safinamide mesilate)’ is expected to be in short supply for the next month, causing inconvenience to patients. In particular, although there are drugs with similar efficacy to Equfina, there are no direct therapeutic substitutes available with the same ingredients. The company reported the supply shortage to the Ministry of Food and Drug Safety on the 21st and announced today (from the 22nd) that a temporary supply shortage of Equfina is expected until August 31. The reason for the shortage is an issue with the release testing. Eisai stated, “Due to issues with the external testing agency, release testing has not been conducted, and a temporary supply disruption is expected once existing inventory is depleted.” Eisai explained, “While there are alternative medications with similar efficacy used for Parkinson's disease treatment, Equfina is a new drug, so there are currently no direct therapeutic substitutes. We will work closely with the testing agency to resolve the supply shortage as soon as possible.” Currently, there are no generic versions of Equfina available in the domestic market. However, companies such as Myung In Pharm, Bukwang Pharmaceutical, and Samil Pharmaceutical filed patent challenges. Myung In Pharm received approval on the 17th to initiate a bioequivalence test for its Equfina generic and is now proceeding with product development. Equfina was approved by the Ministry of Food and Drug Safety on June 24, 2020, as a once-daily levodopa adjunctive therapy for Parkinson's disease patients. Equfina is a new third-generation MAO-B (monoamine oxidase-B) inhibitor that acts on both dopaminergic and non-dopaminergic signaling pathways. In a Phase III clinical trial, it demonstrated significant improvements in both motor and non-motor symptoms in Parkinson's disease patients experiencing motor fluctuations. The current standard of care for Parkinson’s disease is levodopa, but it has been reported that approximately 75% of patients may develop complications after long-term (5 years or longer) use of levodopa. Equfina is used as an adjunctive therapy to levodopa, increasing the duration of action of levodopa, which may decrease with long-term use. Equipina is initiated at a dose of 50 mg once daily, with the option to increase the dose to 100 mg once daily (two 50 mg tablets) based on individual patient response and tolerability. The incidence of adverse reactions following Equipina administration was similar to that of placebo, with most (over 90%) being mild to moderate in severity. Even after two years of long-term administration, it demonstrated a safety profile similar to those observed in previous studies.
- Policy
- Jardiance 10mg price reduced from ₩618 to ₩582
- by Lee, Tak-Sun Jul 21, 2025 06:06am
- The maximum insurance price ceiling for the 10 mg flagship dosage form of the SGLT-2 inhibitor diabetes treatment Jardiance Tab (empagliflozin, Boehringer Ingelheim), will be adjusted as reimbursement will also apply to chronic kidney disease. Jardiance recorded KRW 66.3 billion in outpatient prescriptions (based on UBIST) last year. According to industry sources on the 18th, the insurance price ceiling for Jardiance 10mg will be reduced from KRW 618 to KRW 582 as of August. This is a 5.8% reduction. Along with the price cut, Jardiance 10 mg will also be reimbursed for non-diabetic chronic kidney disease from August. A patient needs to meet all of the following conditions to receive reimbursement for Jardiance 10mg: ▲is on a stable dose of an ACE inhibitor or angiotensin II receptor blocker at the maximum tolerated dose for at least 4 weeks ▲has an eGFR between 20 and 75 ml/min/1.73 m², and ▲urine dipstick test is positive (1+ or higher) or uACR is 200 mg/g or higher. Jardiance is also administered in combination with other standard treatments for kidney disease. Another SGLT-2 inhibitor, dapagliflozin, has also been granted reimbursement for the treatment of chronic kidney disease starting this month. As a result, dapagliflozin-containing drugs and Jardiance, for which no generic versions have been released yet, are now reimbursed not only for diabetes but also for chronic heart failure and chronic kidney disease, greatly expanding their scope of use. In Jardiance’s case, Jardiance Tab 10 tablets will undergo a preemptive price reduction due to its expanded scope of use, resulting in an adjustment of its insurance ceiling price. Originally, the price of Jardiance 10 mg Tab was scheduled to be reduced from KRW 618 to KRW 591 starting in July under the price-volume agreement (Type B, PVA) scheme. However, considering the potential confusion and administrative burden caused by consecutive price reductions, the government decided to adjust the prices unilaterally starting in August. On the other hand, the price of Jardiance 25mg was reduced from KRW 798 to KRW 762 in July under the PVA. Type B of the PVA scheme refers to cases where the insurance price ceiling has been adjusted under Type A, or where the maximum price has not been negotiated under Type A, and the claims amount for the same product group has increased by more than 60% compared to the previous year's claims amount, or has increased by more than 10% with an increase of KRW 50 billion or more, from the date of initial listing or the date the insurance price ceiling was adjusted through negotiation. The insurance price ceiling is adjusted through the company’s price negotiations with the National Health Insurance Service. As Jardiance is a blockbuster drug with annual prescriptions exceeding KRW 60 billion, healthcare institutions are advised to exercise caution with regard to the price reduction. Jardiance is co-marketed in Korea by Boehringer Ingelheim and Yuhan Corp.
- Policy
- [Reporter's View] A separate fund for new orphan drugs
- by Lee, Jeong-Hwan Jul 18, 2025 06:36am
- Will the 'establishment of a separate fund,' considered one of the ways to improve patient access to expensive, rare, and intractable disease drugs, become even more difficult with the inauguration of the Lee Jae Myung administration? Jung Eun-kyung, the candidate for Minister of Health and Welfare (MOHW), who is facing a parliamentary confirmation hearing on July 18, stated that "expanding reimbursement coverage should take precedence over establishing a fund" for drugs exclusively for rare and intractable diseases, separate from the National Health Insurance finances. Jung's statement is interpreted as an intention to maintain the traditional method of determining reimbursement for rare disease patients through national support systems, such as 'special calculation and improvement of standards'and catastrophic medical expense support, within the currently available National Health Insurance finances, rather than creating an additional financial "pocket." This is different from the MOHW's previous stance, where it had agreed with parliamentary legislation aimed at accelerating National Health Insurance reimbursement for high-priced, orphan disease treatments through the establishment of a separate fund. Conversely, candidate Jung showed a completely different attitude regarding the creation of a regional essential medical care fund. Jung presented a clear vision, stating, "If appointed as minister, I will push for the enactment and amendment of relevant laws through consultation with ministries, including the Ministry of Economy and Finance, to ensure the establishment of a regional essential medical care fund." Considering that the frequency of approvals for ultra-high-priced new drugs, such as immunotherapy for cancer and rare disease biologics, has increased incomparably compared to the past, Jung's skeptical expression regarding the establishment of a dedicated fund for rare diseases is disappointing. Suppose expensive new drugs are to be reimbursed within the limited National Health Insurance finances and restricted drug expenditure boundaries. In that case, the responsible authorities will ultimately have no choice but to make innovative efforts to diversify drug reimbursement criteria or to cut drug prices for already approved medicines. Reducing reimbursement for already approved drugs would ultimately increase the likelihood of further drug price reductions, which could lead to protests from domestic pharmaceutical companies and a contraction of funds needed for innovative new drug development. This means that a zero-sum game between multinational pharmaceutical companies, which focus on developing new drugs, and Korean pharmaceutical companies, which have a large proportion of generic products, could intensify over National Health Insurance finances. Therefore, establishing a separate fund for rare diseases is a legislative and administrative task that the new government should proactively consider. The MOHW should not exclude the establishment of a separate fund from its policy priorities. Instead, it should actively engage in administrative efforts, gathering wisdom with the National Health Insurance Service, the Health Insurance Review & Assessment Service (HIRA), and the medical community, to persuade the Ministry of Economy and Finance, which opposes the fund for reasons such as uncertainty of efficacy and fairness with other diseases. The National Health Insurance authorities are the only party that should analyze cases from advanced countries, such as the UK's operation of the Cancer Drug Fund (CDF), to ensure patient access to treatments for rare diseases and strive to open the minds of financial authorities. If rare·intractable diseases and rare disease drugs are translated into English, they become 'orphan diseases' and 'orphan drug.' They are pitiful diseases and treatments, like a child who has lost both parents or been abandoned, with no comfortable place to lean on. Currently, bills establishing separate funds to strengthen national responsibility for orphan diseases and treatments are pending in the 22nd National Assembly. These include the package of bills proposed by Representative Seo Myeong-ok of the People Power Party for the establishment of a Cancer Management Fund·Rare Disease Fund (amendments to the Cancer Management Act·Rare Disease Management Act·National Finance Act·Lottery Tickets and Lottery Fund Act), and the package of bills proposed by Representative Jeon Jin-sook of the Democratic Party of Korea for the expansion of National Health Insurance reimbursement for rare and severe disease treatments (amendments to the National Health Insurance Act·Lottery Tickets and Lottery Fund Act). We look forward to a future where Jung, having passed the confirmation hearing and been appointed MOHW, actively engages in persuasion and consultation with the Ministry of Economy and Finance to ensure the passage of the orphan disease fund establishment bills in the National Assembly. In this case, it would be favorable news for patients and caregivers who suffer from the burden of expensive hospital bills and high-priced treatments, as well as for the National Health Insurance finances, which currently bear a heavy burden.