- LOGIN
- MemberShip
- 2025-12-23 05:36:55
- Policy
- Rozlytrek & Vitrakvi are likely to be listed in April
- by Lee, Tak-Sun Mar 22, 2022 05:54am
- Bayer Korea's Vitrakvi and Roche Korea's Rozlytrek completed drug price negotiations and are likely to register their benefits in April. Both drugs are used to treat solid cancer in adults and children with confirmed NTRK gene fusion. If NTRK gene fusion is confirmed, it can be administered regardless of cancer. According to the industry on the 21st, the two drugs are likely to complete drug price negotiations with NHIS and register their benefits in April. Rozlytrek and Vitrakvi were granted permission in April 2020 and May 2020, respectively. Rozlytrek and Vitrakvi are equally used in adult and pediatric solid cancer treatments with neurotic tyrosine receptor kinase (NTRK) gene fusion. Rozlytrek also has a local progressive or metastatic non-small cell lung cancer indication that is positive for ROS1 in adults. Rozlytrek showed 56.9% ORR in a clinical trial (STARTRK-2) conducted in patients with solid cancer positive for the NTRK fusion gene. More than half of the subjects were confirmed to have decreased tumors. Vitrakvi also showed 75% ORR in clinical trials of 55 people whose NTRK gene fusion was confirmed. The two drugs passed the HIRA's Cancer Disease Review Committee in May last year and were reviewed by the Drug Benefit Assessment Committee in November of the same year. As a result of the deliberation, Vitrakvi recognized the appropriateness of the benefit, but in the case of Rozlytrek, the appropriateness was recognized if the conditions for an additional drug reduction and an appropriate refund rate were accepted. Vitrakvi was also recognized for adequacy, but there was also a clue that Rozlytrek's cost-effectiveness evaluation results need to be further considered. The two drugs with the same efficacy were simultaneously placed on the same line and the evaluation was conducted. Since then, the two drugs have been negotiated with NHIS. However, they failed to reach a conclusion within 60 days, the deadline for negotiations, and began additional negotiations. The NHIS posted on its website that negotiations on the two drugs have been completed. An industry official said, "As announced by NHIS, negotiations on the two drugs have been completed and we understand that they will be reimbursed in April." If the two drugs are reimbursed, it is expected that new treatment benefits will be given to cancer patients whose NTRK gene fusion has been confirmed.
- Policy
- Actemra is likely to be reimbursed for managing CRS
- by Kim, Jung-Ju Mar 21, 2022 05:55am
- Actemra (Tocilizumab 200mg), imported by JW Pharmaceutical and used to treat rheumatoid arthritis, is also expected to be paid for CRS management. The MOHW announced on the 18th some amendments to the "Details on the Application Criteria and Methods of Nursing Benefits" containing such contents. Actemra is a drug approved for adult rheumatoid arthritis treatment, systemic childhood idiopathic arthritis, and multi-articular childhood idiopathic arthritis. CRS develops symptoms due to excessive release of cytokines from immune cells in the process of killing cancer cells. The MOHW plans to recognize benefits when administering Actemra to CRS management. The government plans to conduct an industry opinion inquiry by the 27th with the aim of implementing it on the 1st of next month. Meanwhile, Actemra recently received EUA from the MFDS to be used for the treatment of severe COVID-19 patients over the age of 2. The drug is approved as a COVID-19 treatment in the United States, Japan, and Europe.
- Policy
- In/outpt Rx for Paxlovid are available in nursing hospitals
- by Lee, Jeong-Hwan Mar 21, 2022 05:55am
- Nursing hospitals will be able to prescribe outpatient & inpatient Rx for Paxlovid, a COVID-19 treatment. Both outpatient prescriptions that prepare and supply oral drugs at pharmacies in charge and inpatient prescriptions that receive oral drugs from hospitals dedicated to infectious diseases that supply treatments have become possible. The KDCA made the announcement at a regular briefing on the 17th. The KDCA judgment that group infections in nursing hospitals are continuing and timely administration of PO treatments for the elderly affected the permission of outpatient & inpatient prescriptions in nursing hospitals. Kim Ok-soo, head of the resource management team at the quarantine countermeasures headquarters, explained, "The nursing hospital originally tried to prescribe Paxlovid as outpatient Rx, but there was a shortage of supplies at pharmacies in charge of cities, counties and districts. Team leader Kim Ok-soo added, "The nursing hospital has improved the system since the 14th so that inpatient and outpatient Rxs can be made in a timely manner," adding, "It is a new application." Since the 14th, KDCA has taken measures to prescribe Paxlovid, a treatment to be taken when training rapid antigen tests, for those aged 60 or older, and it is necessary to secure the amount of treatment. According to the KDCA, Paxlovid for 163,000 people was supplied to Korea as of the 16th, and the inventory was 96,000 people. In addition, it is scheduled to be introduced in Korea at the end of March. The KDCA said, "We will promote the prevention of seriousness and the burden of the medical system through active administration to the elderly," adding, "We will ensure that timely administration of treatments can be made."
- Policy
- Oral myeloid leukemia treatment Onureg to be soon approved
- by Lee, Hye-Kyung Mar 18, 2022 05:56am
- The domestic marketing authorization for BMS’s acute myeloid leukemia (AML) treatment, ‘Onureg tablet (azacytidine).’ Is imminent. The safety and efficacy review for the marketing authorization of Onureg, which had received FDA approval in 2020, is now complete in Korea. According to industry sources on the 16th, the Ministry of Food and Drug Safety has completed verification on the efficacy of Onureg that BMS Korea had submitted an application for. With the safety and efficacy review complete, experts expect Onureg, the first oral azacytidine, to be released within the first half of this year. The AML treatments currently approved in Korea are Celgene’s ‘Vidaza inj.100mg,’ Boryung Pharmaceutical’s ‘Vizadakin Inj.,’ Samyang Holdings’ ‘Azalid inj. 100mg,’ and ‘Azalid inj. 150mg.’ All of the motioned drugs are injection types, therefore, if approved, Onureg will become the first oral treatment formulation to be approved in Korea. Hypomethylating agents, which are also known as CC-486, are used to treat adult AML patients who achieved CR or CRi following induction therapy with or without consolidation treatment and who are unable to complete intensive curative therapy such as HSCT. Warning and precaution for Onureg include risks of substitution with other azacitidine products, myelosuppression, increased early mortality in patients with myelodysplastic syndromes, and embryo-fetal toxicity.
- Policy
- EUA approval of Actemra for severe COVID-19 over 2 yrs
- by Lee, Hye-Kyung Mar 17, 2022 05:58am
- Actemra can be used in severe COVID-19 patients over the age of 2. The MFDS (Director Kim Kang-rip) announced on the 15th that it approved EUA of Actemra imported by JW Pharmaceutical for severe COVID-19 patients aged two or older to prevent a shortage of treatments in advance. The seriousness referred to herein refers to hospitalized patients who are receiving systemic corticosteroid and need oxygen therapy. The MFDS comprehensively reviewed related data and results of consultation with infectious medicine specialists, and decided EUA after deliberation by the Public Health Crisis Response Medical Product Safety Management and Supply Committee. Actemra is effective in treating severe COVID-19 patients and is used with EUA (USA) or permission (Europe, Japan). Actemra is an antibody drug (more than 60 minutes of intravenous administration) that has already been licensed and used as a treatment for rheumatoid arthritis in Korea. The MFDS said, "We will continue to do our best to quickly supply safe and effective products to overcome COVID-19 and restore the people's daily lives."
- Policy
- Kymriah's price negotiation has rarely been concluded
- by Lee, Jeong-Hwan Mar 17, 2022 05:58am
- Two months after passing the Drug Benefit Evaluation Committee, negotiations on drug prices for acute lymphocytic leukemia and lymphoma CAR-T treatments have rarely been concluded. Leukemia patients are urging the NHIS and Novartis Korea to strengthen Kymriah's access to patients with rapid drug price negotiations. On the 15th, Korea Leukemia Patients Organization pointed out, "Kymriah, which received marketing approval from the MFDS, passed the committee on January 13, but patients are suffering because drug price negotiations have not been concluded." Kymriah's price negotiations will begin on January 27 this year, with the deadline of March 28, considering the 60 days of negotiations. The problem is that even if negotiations have been completed so far, it is difficult to propose the Health Insurance Policy Deliberation Committee in March, and health insurance treatment of Kymriah is only possible in May when it is proposed in April. Patients who need Kymriah treatment are patients with recurrent or refractory acute lymphocytic leukemia and lymphoma without treatment, and the life expectancy is less than six months. With one treatment, Kymriah demonstrated long-term survival in 8 out of 10 patients with acute lymphocytic leukemia and 4 out of 10 patients with lymphoma. Kymriah's one-time non-reimbursed costs about 460 million won, with health insurance authorities and Novartis unable to reach a deal. After passing the Cancer Disease Review Committee and the Pharmacist Evaluation Committee, patients are appealing for a negotiation through mutual efforts between the NHIS and pharmaceutical companies. Patients suffered a lot of chemotherapy and hematopoietic stem cell transplants due to recurrence several times, the association said. "The reason why they are waiting for the one-shot treatment Kymriah is that they can no longer handle the pain." The association said, Kymriah has been registered for registration for one year, he said. "If the registration is delayed because the drug price is expensive and the number of patients is small, it is the same as denying the existence of health insurance." He then said, "We should not restrict access to Kymriah, which is much more effective than existing treatments, because it is an advanced bio-new drug." "Over the past year, about 200 leukemia and lymphoma patients have given up on Kymriah treatment and most have died. If health insurance is not applied from April after concluding drug price negotiations, a free system should be implemented."
- Policy
- Expectations on Yoon Seok Yeol's government
- by Lee, Jeong-Hwan Mar 16, 2022 05:57am
- Expectations are growing for the Yoon Seok-yeol administration's policy to quickly register and expand benefits for ultra-high-priced medicines such as anticancer drugs and rare incurable disease drugs. This is the effect of Yoon Seok-yeol, the elected president of the People's Power, making a pledge to quickly register severe diseases and rare cancers and expand health insurance to reduce the burden on patients and insurers. On the 15th, pharmaceutical companies and patient groups are paying attention to the trend of changes in health insurance policies for rare and intractable diseases that have obtained domestic marketing permits. Attention is focusing on whether cases in which health insurance registration and drug price negotiations have been relatively delayed within the Moon Jae In government period can be resolved with the inauguration of the new government. Yoon has put the rapid registration of rare and intractable disease drugs and the expansion of health insurance at the forefront of the campaign. Specifically, Yoon criticized the current government for being too slow to register new drugs for cancer and rare incurable diseases. It was pointed out that the Health Insurance Review and Assessment Service should register health insurance within 180 days, with 120 days for drug adequacy evaluation and 60 days for the National Health Insurance Service. It also said that although the RSA was introduced in December 2013, it was applied only to 41 drugs, and 32 were focused on cancer diseases as anticancer drugs. At the same time, Yoon announced that he would shorten the health insurance registration process for anticancer drugs and severe disease treatments without alternative drugs. It said it will significantly reduce the number of days listed by allowing post-evaluation and drug price negotiations to be conducted at the same time when conditions are met after pre-evaluation by the Korea Appraisal Board, and increase the drug price negotiation rate by using RSA of rapid registration. Pharmaceutical companies and patient groups are predicting a paradigm shift in the new government's policy to register new drug health insurance. The KRPIA, which met with party leader Lee Joon-seok just before the presidential election, also urged the establishment of a new health insurance and drug price system that can revitalize the use of innovative new drugs that are effective but expensive. The KRPIA criticized the narrow coverage of the current RSA and demanded that RSA be applied to drugs that are recognized for insurance fiscal neutrality and therapeutic needs, as well as drugs other than anticancer drugs and rare diseases. The patient group also delivered the "introduction of a rapid registration system for new drug health insurance directly related to life" as the top policy proposal in the four patient policies desired for the presidential candidate for the new year. Attention is focusing on how much the Yoon Seok-yeol administration can realize such demands for improvement in health insurance policies by the pharmaceutical industry and patients. An official in charge of drug prices belonging to a global pharmaceutical company said, "It is natural to expect the introduction or conversion of a new new drug health registration policy because the regime has been replaced. We hope that a different track from the current government or a more flexible health insurance and drug price system will be introduced. We need to actively come up with new tools to evaluate the value at a time when ultra-high-priced new drugs are pouring out."
- Policy
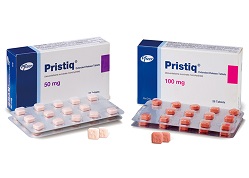
- Price of desvenlafaxine IMDs to be discounted up to 10%
- by Lee, Tak-Sun Mar 16, 2022 05:57am
- Pic of the anti-depressant ‘desvenlafaxine’ The price of the follow-on drugs of the anti-depressant ‘desvenlafaxine’ that had been listed at 90% of the price of its original by avoiding the original’s patent through salt alterations have fallen greatly after a year and a half due to the adjustment of the price cap that was conducted following an investigation into their actual transaction price. In other words, the companies’ efforts to develop formulations to receive a higher drug price were all in vain. Four companies including Hanlim Pharm, Myung-In Pharm, Nexpharm, and Whan In Pharm had first received approvals for their incrementally modified desvenlafaxine drugs on April 7th, 2020. The four drugs were all listed for reimbursement on June 1st, 2020. The original desvenlafaxine product is Pfizer Korea’s ‘Pristiq ER (desvenlafaxine succinate monohydrate).’ The patent for Pristiq ER will expire on October 7th this year, but the 4 companies had developed a salt-modified version that differs from the original and succeeded in avoiding the original’s patent. This allowed the companies to release their products before the patient's expiry term. In addition, the companies received a price that was set at 90% of the original based on the formula for calculating the drug price of a salt-modified drug before the expiration of the original’s patent. Based on June 1st, 2020, Pfizer’s Pristiq ER 100mg was priced at ₩1,257, while other salt-modified products were set at ₩1,129. The price difference between the two is only ₩128. However, due to the aftermath of the investigation into the drugs’ actual transaction price and the price cap adjustments that followed, the price gap had increased by 10% to 20% at the most. Myung-In’s Esven SR that was priced at ₩1,129 per 100mg fell to ₩1,016, Hanlim’s Prinexor ER to ₩1,050, and Whan In’s Defaxine SR to ₩1,016. Nexpharm Korea’s Desvela was the only drug that was able to maintain its original price of ₩1,129. Myung-In, Hanlim, and Whan In’s drug prices fell 7.0%, 10.0%, 10.0% respectively after the price cap adjustments that were applied following an investigation into their actual transaction price. The actual transaction price of Pfizer Korea’s Pristiq ER 100mg had also been adjusted following investigations, but its price drop was a mere 0.3% and was set ₩4 lower at ₩1,250. In other words, the price of salt-modified drugs has fallen 7-10% in just a year and a half. As their higher drug price was set as compensation for developing ingredients that can avoid the original's patent, the disappointment on the companies’ part is expected to be large. On the other hand, the question of why the drug prices were set at a higher level than the actual transaction price in the first place may also arise.
- Policy
- HPV vaccine, which costs 600,000 won, free of charge ?
- by Lee, Jeong-Hwan Mar 16, 2022 05:57am
- Human papilloma virus (HPV) vaccine, which costs about 200,000 won per inoculation and 600,000 won per inoculation, is expected to do well in the health insurance coverage of room 9. It is the gate that President-elect Yoon Seok-yeol promised to expand the insurance of Gadasil 9 as a life-friendly pledge. Looking at the 13th "59-second shorts" pledge posted on Yoon's YouTube channel on the 10th, the cost of vaccination for MSD Gadasil 9 in Korea, called cervical cancer vaccine, is expected to increase. Gadasil 9 currently has a much wider range of prevention of related diseases than Cervarix and Gardasil, the national HPV vaccines. This is because Cervarix is a divalent and Gardasil is a tetravalent vaccine, which has fewer target viruses than the 9-valent Gardasil 9. As Gardasil 9 targets a wider range of disease-causing viruses, inoculation costs are also high. Currently, the price of vaccinations for Gardasil 9 at front-line medical institutions is between 450,000 won and 600,000 won. In the 59-second shorts pledge, Yoon promised to apply insurance to both the recommended age and gender of Gardasil 9 licensed in Korea. Specifically, Gardasil 9 inoculation age is 9 to 45 years old for women and 9 to 26 years old for men, and Yoon pledged to pay all of them for inoculation. Women can prevent cervical cancer, vulva cancer, vaginal cancer, anal cancer, genital warts, etc. caused by HPV infection through Gardasil vaccination, and men can prevent anal cancer and genital warts caused by HPV infection. HPV is a virus that spreads to women through men and requires vaccination for both men and women, but the male vaccination rate has been significantly lower than that of women. Gardasil 9 is inoculated three times, and the price is known to be approximately 500,000 won to 700,000 won. As a result, the possibility of Gardasil9's health insurance benefits is expected to increase significantly after Yoon took office as president. It is explained that the People's Power Party fully considered the budget for Gardasil 9 health insurance benefits at the time of designing the pledge. "Gardasil 9 is inoculated to both women and men and must be inoculated three times in total," said an official at the People's Power Election Headquarters. "The pledge is to support the cost of inoculating 200,000 won per session three times."
- Policy
- The government will begin to establish an Asian vaccine fund
- by Lee, Jeong-Hwan Mar 15, 2022 05:58am
- The government will review the need for joint vaccine purchase funds in Asia and start working on creating grounds for establishment. The move is aimed at preventing repeated cases of global difficulties in supplying vaccines with COVID-19 Pandemic and bridging the gap in accessibility to public vaccines and biopharmaceuticals by country. On the 14th, the Ministry of Health and Welfare's Global Vaccine Hub Promotion Team announced that it will start researching a joint vaccine purchase fund to ensure access to vaccines in mid- to low-income countries in Asia. According to the MOHW, GAVI had difficulty in supplying vaccines in Asia as it implemented a vaccine supply policy that utilizes a differentiated pricing system according to income by country. Accordingly, the background of this study is that it is necessary to prepare to become a global vaccine hub by promoting vaccine cooperation in Asia. The Ministry of Health and Welfare predicted that through research, large-scale vaccine demand will be discovered, Korean vaccine production companies will pioneer markets and lay the foundation for vaccine exports by the authority to adjust the fund's contribution. It also analyzed that it will secure equity by bridging the gap in accessibility between countries to global health crisis management such as Pandemic and essential public vaccines and biopharmaceuticals. The Ministry of Health and Welfare will review the need for a joint Asian vaccine purchase fund and conduct literature surveys and interviews to lay the groundwork for its establishment. Considering the national mandatory vaccination by country and the status of infectious diseases, the status and demand of inconsistency in supply and demand of vaccines will be identified. Specifically, the current system to strengthen vaccine accessibility in middle and low-income countries is analyzed, and the necessity of introducing a vaccine accessibility enhancement program for countries excluding GAVI is reviewed. It will also come up with a plan to establish a vaccine joint purchase fund. Starting with the analysis of the impact of GAVI vaccine supply mechanisms and changes in target countries on vaccination by country, the need for Asian vaccine accessibility programs such as joint Asian vaccine purchase funds will also be reviewed. Along with basic designs such as vaccine joint purchase fund operators, joint purchase items, and operating systems, it will also investigate whether it can function as a basis for Korean vaccine exports by utilizing coordination rights based on fund establishment such as specific company priorities. The MOHW said, "It takes time to establish a vaccine joint purchase fund and it will estimate the budget and set the scope of participating countries, regions, and private foundations. The MOHW will visit the SAEAN to exchange information and ask for cooperation." The MOHW said, "We will be able to establish policies based on empirical data through accurate diagnosis and analysis related to the establishment of the Asian Vaccine Joint Fund." The Ministry of Health and Welfare added, "We will present the direction of fund establishment according to the demand and supply of vaccines for major infectious diseases by country and design effective operation plans."