- LOGIN
- MemberShip
- 2025-12-23 07:14:45
- Policy
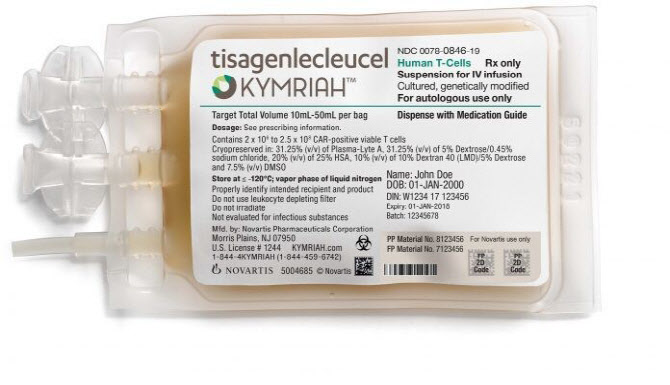
- Kymriah has been listed, hope of cure
- by Lee, Jeong-Hwan Apr 05, 2022 05:57am
- Patients immediately welcomed the confirmation of health insurance benefits of the acute lymphocytic leukemia and lymphoma CAR-T treatment Kymriah (Tisagencleucel). The patients urged the transition committee of the next presidential post and Yoon Seok-yeol to quickly register new drugs directly related to life as health insurance benefits. On the 31st, Korea Leukemia patient organization said, "The cost of one-time non-reimbursement payment is 460 million won, and the ultra-high-priced Kymriah passed the Health Policy Review Committee and acquired health insurance benefits to even hope for cure." Kymriah will be listed with an upper limit of 360 million won for one-time health insurance from the 1st. The Korea Leuchemia patient organization believes that the appeal of Cha Eun-chan's mother, Lee Bo-yeon, who left for heaven while preparing for Kymriah treatment, and the consent of tens of thousands of Cheongwadae petitioners influenced Kymriah's health insurance registration in a year and a month. The National Human Rights Commission of Korea expressed its opinion to the MOHW to establish a rapid registration system for Kymriah's health insurance system, which was raised by the Korea Leukemia patient organization, also positively affected benefits. The Korea Association of Patients urged the government to manage the risk sharing based on patient-level performance-based risk sharing that applies only to lymphoma, as well as Refund risk sharing and Extended Cap risk sharing, which are conditions for health insurance registration for Kymriah. The Association of Patients argued, "The next presidential transition committee and the Yoon Seok-yeol government should also introduce a rapid registration system for new drugs that show excellent effects without alternative drugs for life-threatening patients such as Kymriah."
- Policy
- Phase 3 of Jetema the toxin will be conducted in Korea
- by Lee, Hye-Kyung Apr 04, 2022 06:07am
- Jetema the toxin 100U, which has been approved for export botulinum toxin, will conduct phase 3 clinical trials in Korea. The MFDS recently approved Jetema the toxin for a "multi-organization, double-blind, random assignment, parallel design, noninferiority trials, active control groups, and phase 3 clinical trials" for JTM201. The clinical trial will be conducted at Asan Medical Center in Seoul, Konkuk University Hospital, and Chung-Ang University Hospital. Jetema is conducting a clinical trial of Jetema the toxin after obtaining a drug manufacturing license in January 2019, and is expected to officially apply for an item license in Korea when the phase 3 clinical trial is completed. In June 2020, Jetema received permission from the MFDS to export Jetema the toxin for full-fledged overseas expansion and commercialization. It signed a local clinical and supply contract worth 144 billion won with Brazil even before applying for clinical trials and acquiring KGMP, and signed an exclusive sales and supply contract with HUADONG MEDICAL AESTHETICS as a Chinese market supply partner in February this year. Jetema aims to conduct phase 1 of Jetema the toxin in China by the end of this year along with phase 3 clinical trials in Korea. Since the beginning of this year, news of phase 3 clinical trials for botulinum toxin, a treatment for improving wrinkles in adults, has continued in Korea. On the 1st, the phase 3 clinical trial of Protox, a botulinum toxin A drug applied by the MFDS, was approved. Clinical trials of Protox are conducted at Chung-Ang University Hospital.
- Policy
- 20 ultra-high-priced drugs over ₩5 mil sold in Korea
- by Lee, Tak-Sun Apr 04, 2022 06:07am
- Survey results have shown that 20 high-priced drugs over ₩5 million are approved for reimbursement 10 years after, Soliris, which had been then the most expensive drug in the world, was listed for reimbursement in Korea With ‘Kymriah,’ the one-shot treatment that was listed for reimbursement on April 1st, recording the highest price at ₩360,030,000, the top 4 drugs with regards to their price were listed by the Moon’s administration. Looking at the price caps set in the reimbursement list, a total of 20 drugs were found to cost over ₩5 million. When lowering the standard to ₩10 million, the number of drugs increased to 84. The drug in 50th place is the PNH treatment Soliris which was listed 10 years ago in 2012. At the time, Soliris’s price was set at ₩5 million per year, sparking heated debate on the reimbursement of the drug. However, 49 more drugs with a higher price cap had been introduced to the market in 10 years. Of course, the price cap cannot determine the cost of each drug as their treatment period differs. For example, Kymriah is administered once in a lifetime, and in this sense, the drug’s ₩360,030,000 is cheaper than the ₩500,000,000 that was set as the annual cost for Soliris 10 years ago. By price cap alone, Kymriah is the only drug priced at the 100 million level. The second in line is the SMA treatment Spinraza, which costs ₩92,350,000. In third place is the neuroendocrine tumor treatment Lutathera set at ₩22,100,000, followed by the immunotherapy cancer drug Yervoy set at ₩14,000,000. Fourth is the stem cell therapy for Crohn’s Fistula, Cupistem at ₩13,490,000. What is unusual is that the top 4 most-expensive drugs on the list were approved for reimbursement under Moon’s administration. As new drugs with better efficacy in rare diseases are being introduced, the fact that the latest drugs have a higher price is, in a sense, natural. Spinraza was listed for reimbursement in April 2019, Yervoy in September last year, Lutathera from March this year, and Kymriah from April, after which the NHI will be supporting most of their costs. Such essential drugs that are ultra-high-priced will continue to be released in the future. The increased number of such drugs can also burden NHI finances, therefore, the government’s concern over effective fiscal sharing will also continue to deepen.
- Policy
- Based on PVA exclusion, the arithmetic average is 90%
- by Lee, Tak-Sun Apr 04, 2022 06:07am
- Drugs subject to PVA with an arithmetic average of less than 90% of the same product group are excluded. Previously, only drugs below the arithmetic average were excluded, but the target was further narrowed to less than 90%. Products with annual claims of less than 2 billion won are also excluded from PVA drugs. Previously, products worth less than 1.5 billion won were excluded, but the revision will expand the drugs subject to exclusion. The NHIS announced on the 28th that it will revise detailed operating guidelines for PVA negotiations to enhance the role of drug expenditure management and promote efficiency in operating the system. The NHIS explained that the PVA negotiation system is a system that adjusts drug prices as the usage increases after listing drugs, and plays a key role in the follow-up management of drug prices. The need to improve effectiveness has been steadily raised, and the guidelines have been revised this time. The revision of the guidelines focused on revising the drugs subject to negotiation (Article 6 of the Guidelines) for efficient operation of the system and financial management. First, in order to select the top drug of the amount of claims excluded due to reasons below the arithmetic average, the rule excluding "less than the arithmetic average" was revised to the "less than 90% arithmetic average" rule. As of 2020, the average claim for drugs subject to PVA negotiations in 2021 was 12.7 billion won, while those subject to exclusion below the arithmetic average were 22.3 billion won, much more than this. Most of the drugs subject to exclusion below the arithmetic mean were between 90% and 100% of the arithmetic mean. An official from the NHIS explained, "In the past, it was difficult to efficiently operate the system by excluding drugs below the arithmetic average price from the PVA negotiations." The revision will revise the existing regulations to less than 2 billion won in order to exclude small claims with low fiscal impact from negotiations. Among the subjects of the 2021 negotiations, drugs in the section between 1.5 billion won and 2 billion won in claims account for 35.6% of the total. Jeong Hae-min, head of the NHIS' Pharmaceutical Management Office, explained, "The revision of the detailed operation guidelines for PVA negotiations will strengthen the follow-up management of drugs that affect insurance finances by strengthening drug management." The revised detailed operation guidelines for PVA negotiations will be implemented from April 1, and they will also be applied to drugs undergoing PVA monitoring and negotiations at the time of implementation of the guidelines.
- Policy
- To commercialize innovative new drugs/supply essential drugs
- by Lee, Jeong-Hwan Apr 03, 2022 04:25pm
- The MFDS reports discuss ways to become a bio-health powerhouse The Presidential Acquisition Committee Yoon Seok-yeol and the MFDS agreed to systematically support the commercialization of high-tech and innovative medical products and establish a stable supply environment for rare essential drugs with low profitability. The transition committee plans to take the lead in the development of domestic treatments for COVID-19 to establish sovereignty over vaccines and treatments, a major pledge of Yoon Seok-yeol. On the 28th, Yoon Seok-yeol's transition committee's social welfare and culture division made the announcement after reporting to the MFDS. Lim Yi-ja, secretary-general of the MFDS, as well as standing expert committee members Kim Mi-ae and Seo Jung-sook of Ahn Sang-hoon, Baek Kyung-ran, and Kim Do-sik were present in the report. From the MFDS, deputy director Kim Jin-seok, evaluation director Seo Kyung-won, and directors attended. The development of domestic treatment for COVID-19 is a major pledge related to COVID-19 by President-elect Yoon Seok-yeol. Yoon promised to expand full national R&D support to establish sovereignty over vaccines and treatments and build a global vaccine hub. It will also come up with policies to open Yoon's pledge, and the MFDS emphasized the importance of ▲preemptive preparation of predictable screening criteria,▲ systematic commercialization support for advanced and innovative medical products, ▲ training of human resources with global level of regulatory response capabilities, and▲ the role of the MFDS to leap forward as a bio-health powerhouse, such as strengthening international cooperation to secure global competitiveness. Discussions on preparation for the outbreak of new infectious diseases were also held. The acquisition committee and the MFDS agreed that rare and essential medical products, which are difficult to supply to the market due to lack of profitability, should play their role in the country. The transition committee said, "We need to discuss with various experts and closely cooperate with related ministries in the stage of reviewing the safety and effectiveness of medical products." The transition committee said, "We should respond quickly with forward-looking judgments in crisis situations such as COVID-19. The MFDS should make efforts to ensure that domestic medical products have international competitiveness."
- Policy
- The MFDS released a national lot release of Comirnaty
- by Lee, Hye-Kyung Mar 31, 2022 04:29pm
- The MFDS (Director Kim Kang-rip) announced on the 29th that it has released 299,000 doses of Pfizer's Comirnaty 0.1mg/mL (for 5-11 years old) in Korea. The MFDS conducted Comirnaty test and reviewed the manufacturing and test data of the manufacturer, and confirmed the effectiveness, safety, and quality, and decided to release the national lot according to the standards. Comirnaty 0.1mg/mL (for 5-11 years old) is an mRNA vaccine developed and produced by Pfizer of the United States for the purpose of preventing COVID-19 between the ages of 5 and 11. The previously approved Comirnaty, Comirnaty 0.1 mg/mL and Tozinameran were the same active ingredient, but the dose was reduced to 1/3 (10)) per inoculation. The MFDS expected this national lot release to help prevent children from getting worse due to COVID-19 and seriousness at a time when the number of confirmed children increases and family infections increase. COVID-19 vaccine national lot release information can also be found online on the MFDS' website, COVID-19 Vaccine and Treatment Information (www.mfds.go.kr). The national lot release is a system in which the state comprehensively evaluates the test results and manufacturing and test results for each manufacturing unit (lot numbers) before vaccines are distributed on the market.
- Policy
- Roche RET target anticancer drug Gavreto, approved in Korea
- by Lee, Hye-Kyung Mar 31, 2022 04:23pm
- Roche's non-small cell lung cancer treatment Gavreto (Pralsetinib)"has obtained an item license in Korea. The MFDS approved Gavreto 100mg on the 29th. The drug was recognized for its effectiveness in the treatment of ▲RET (RET) fusion-positive local progression or metastatic non-small cell lung cancer adult patients and ▲ systemic therapy required RET mutation local progression or metastatic thyroid medullary cancer for adult patients. The effectiveness is based on the reaction rate and reaction period, and there is no data proving the improvement of the survival period. According to 136 patients who have been administered platinum-based chemotherapy drugs and have been administered Gavreto, the total response rate was 58.8% and the average response duration of 22.3 months. The recommended dosage is 400 mg in adults, which is administered orally once a day. Gavreto obtained conditional approval from the EU Commission in November last year after receiving accelerated approval from the US FDA in September 2020. The FDA approved adult metastatic RET gene fusion-positive non-small cell lung cancer treatments and progressive RET mutation thyroid cancer treatments for children and adults over the age of 12, and the EU applied for permission as a treatment for RET mutated thyroid cancer. Competitive drugs are Lilly's Retevmo 40mg and Retevmo 80mg, and Retvmo was approved from the MFDS on March 11. Retvmo is the first FDA-approved RET inhibitor in May 2020. In Korea, it has indications such as metastatic RET fusion-positive non-small cell lung cancer, progressive or metastatic RET-variant thyroid medullary cancer requiring systemic therapy with previous treatment experience of Sorafenib and/or Lenvatinib. Among domestic pharmaceutical companies, HK inno.N introduced the RET target anticancer drug "VRN061782" from Voronoi in February last year and is conducting commercialization development.
- Policy
- Reimbursement priorities in ultra-high-priced one-shot Txs
- by Lee, Tak-Sun Mar 31, 2022 05:57am
- With the reimbursement imminent for the ultra-high-priced one-shot treatment Kymriah, the National Health Insurance Service is preparing to conduct research on the performance evaluation of the risk-sharing agreement (RSA) and its mid-to-long-term development direction. The research will be exploring ways on the development direction of the RSA system through measures such as setting priorities in the reimbursement of ultra-high-priced one-shot treatments, etc. On the 28th, the NHS made an urgent announcement for a bid on research services for the ' Performance Evaluation of the Risk Sharing Agreement Scheme.’ The research is expected to be completed by November this year after signing a research service agreement in April. In the bid proposal document, NHIS stated, “8 years have passed since the introduction of the risk-sharing system in 2014 to improve access to drug treatment, with no objective evaluation of the effect of the introduction of the system. Also, concerns about the sustainability of insurance finances and the function of v are growing due to the rising demand for reimbursement of ultra-high-priced drugs (one-shot treatments) that can cure patients with a single administration,” indicating the need for research. The purpose of the research is to evaluate the performance of the RSA scheme from a social, economic, and industry aspect and conduct an interview with expert groups to analyze the payable price level for ultra-high-priced drugs, to seek direction on the development of the RSA scheme. To evaluate the performance of the RSA, the research will ▲evaluate the social effect of the RSA scheme (comparison of new drug listing rates pre-and post-implementation to evaluate its influence on patient accessibility, and speed of new drug introduction in Korea) ▲evaluate the economic effect of RSA (assessment of fiscal impact through analysis of RSA drug claims data, assessment of its impact in reducing patient burden, etc.) ▲evaluate the industrial effect of RSA (changes in industry activities in the pharmaceutical industry due to introduction or change of the system, etc.) ▲evaluate the effect of introducing the system from various aspects (including collecting opinions from stakeholders such as academia, patients, medical circles, industry, and government). In order to derive mid-to-long-term improvement plans for the development of the RSA scheme, the research will ▲analyze the pros and cons of the system through a performance evaluation and seek development plans ▲seek improvement plans by examining its operations in other countries ▲seek measures to relieve the administrative burden that increased due to RSA follow-up management such as refunds, etc. In addition, to set priorities for reimbursement and wet standards for the willingness to pay on ultra-high-priced drugs, the research will ▲analyze the reimbursement listing and claims status of ultra-high-priced drugs through an operational definition of the drugs ▲ conduct a focus-group interview (FGI) to survey the experts’ will to pay for ultra-high-priced drugs and seek advice on the considerations that should be made for their reimbursement to set priorities in reimbursement. The NHIS plans to use the research results as evidence for coverage reinforcement policies. In particular, when reviewing the reimbursement listing of ultra-high-priced one-shot drugs that have high social demand, the government will be reflecting the reimbursement priorities’, intention to pay, and other criteria derived from the research into their decision-making process. In addition, the goal is to establish a sustainable management system by preparing effective measures to reduce administrative burdens in the post-management of RSA drugs.
- Policy
- Multidrug-resistant ‘Zavicefta’ applies for approval
- by Lee, Hye-Kyung Mar 29, 2022 05:54am
- Pfizer Korea has applied for the approval of its important treatment for severe gram-negative bacterial infection, ‘Zavicefta.’ Zavicefta, which received marketing authorization in 2016. is a combination drug that contains the third-generation ceftazidime. According to industry officials on the 28th, Pfizer Korea recently submitted an application for the marketing authorization of Zavicefta to the Ministry of Food and Drug Safety. Zavicefta was developed to address the urgent need for new antibiotics in severe infections that cause serious problems such as multidrug-resistant pseudomonas aeruginosa or carbapenem-resistant gram-negative Enterobacteriaceae and extended-spectrum-lactamase (ESBL) producing intestinal bacteria. Zavicefta is used for the treatment of adult patients suffering from complicated intra-abdominal infections (cIAI); complicated urinary tract infections (cUTI), including pyelonephritis; hospital-acquired pneumonia (HAP); and the treatment of aerobic Gram-negative infections in adult patients who have limited treatment options. Zavicefta was developed by AstraZeneca. On August 24th, 2016, the company sold the global development and sales rights for its low molecular weight antibiotic business outside the United States to Pfizer.
- Policy
- Will the migraine tx Reyvow step on the same track
- by Lee, Tak-Sun Mar 29, 2022 05:54am
- IldongIldong Pharmaceutical's domestic copyrighted migraine drug Lasmiditan seems to be speeding up its benefit using the patent-approval linkage system. According to the industry on the 28th, the HIRA is recently listening to opinions on whether the migraine treatment Reyvow 50 & Reyvow100mg is eligible for medical care benefits through related conferences. Reyvow is known as the brand name of Lasmiditan, which Ildong Pharmaceutical secured domestic copyright in 2013. The group signed a development partnership and a domestic sales license agreement with CoLucid of the United States, the original developer of the drug. In 2017, CoLucid was acquired by global pharmaceutical company Eli Lilly also holds Lasmiditan's global rights. Reyvow was approved by the U.S. FDA in October 2019, and then Ildong started a bridging study for domestic permits. In January, the MFDS was found to have ended Reyvow's safety and effectiveness review. As a result, it is understood that the screening is underway using the patent-approval linkage system. It is a system that allows the MFDS to apply for a benefit decision before marketing approval based on this data when safety and validity screening are completed. Usually, it is applied after approval from the MFDS, but using the patent-approval linkage system can shorten the evaluation period by 30 to 60 days. Ildong plans to sell Reyvow domestically this year. Reyvow is the first migraine treatment to act on serotonin (5-HT) 1F receptors and has a mechanism to lower neuropeptide release and inhibit pain delivery pathways, including trigeminal nerves. It acts selectively on serotonin receptors and is characterized by no cardiovascular side effects due to vasoconstriction.