- LOGIN
- MemberShip
- 2025-12-23 02:31:23
- Policy
- ↑Rosuvastatin when combined with Brilinta/Rosuvastatin
- by Lee, Hye-Kyung Jul 11, 2022 06:04am
- According to a recent review of EMA safety information, the MFDS decided to establish a new clause on the interaction that combination of Ticagrelor and Rosuvastatin can affect kidney excretion and increase the risk of Rosuvastatin accumulation. According to the new clause, the exact mechanism of action for side effects was not known, but combination of Ticagrelor and Rosuvastatin resulted in decreased kidney function, increased CPK levels, and rhabdomyolysis. Ticagrelor, including Brilinta, is an ingredient that has been approved as a Ticagrelor in Korea. There are 76 items including 60 mg inno.N, Ahngook Ticagrelor, Brigrelor, Boryung Ticagrelor, Ticavix, Tirelor, Ticagrin, Hurorinta, Ticagen, and Brelor. The full-fledged launch is November, when Brilinta's material patent expires, and 25 items, including Boryung Ticagreler, have acquired generic for exclusivity from November 21 this year to August 20 next year. Brilinta, a representative item of Ticagrelor, was licensed for a new drug in 2011 to reduce the incidence of thrombotic cardiovascular events. The MFDS plans to change the permission after conducting an opinion inquiry by the 22nd.
- Policy
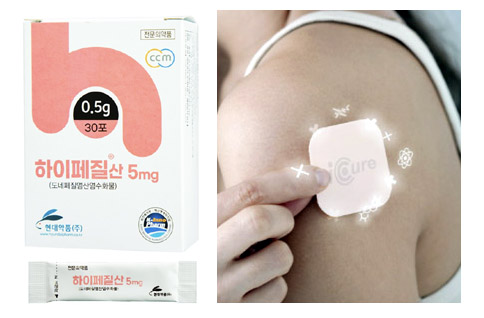
- New Donepezil & a dilemma over low drug prices
- by Lee, Tak-Sun Jul 11, 2022 06:03am
- Hypezil Powder 5mg (left) and IcureDomestic pharmaceutical companies are releasing a new form of Donepezil, a component of dementia treatment, but existing low-cost drugs are acting as a dilemma. Donepezil accounts for about 80% of dementia treatments, so competition among the same products is fierce. As there are 150 pharmaceutical companies with the same ingredient products, the drug price is breaking the lowest price every time. According to the industry on the 7th, Hyundai's Donepezil, Hypezil Powder 5mg, has been applied to the market since this month. This product is expected to be useful for patients who have difficulty swallowing existing tablets because it can be taken in water. The upper limit is 1,700 won. The MFDS recognized this product as a new formulation and approved it as a drug that submitted data. Therefore, if the drug price is calculated based on the standard for calculating the drug price, there is a possibility that the upper limit amount will be determined at 90% of the highest price of the existing product with the same ingredient. As the highest price of Donepezil 5mg is 2,060 won, 90% of this price is 1,854 won. However, Hyundai voluntarily lowered the price from the calculation standard and registered it as 1,700 won. In fact, 1700 won is also a high price based on the Donpezil 5mg. Because the current lowest price is 500 won. As 127 pharmaceutical companies compete for Donepezil 5mg alone, the lowest price is breaking every time a generic comes out. Therefore, it is analyzed that Hyundai, which is releasing new products, had no choice but to take into account the price competitiveness of the Donepezil market. Among the new Donpezil formulations, the patch system is expected to be released as early as the next day. This is because Donerion Patch (Celltrion) was conditionally passed by the Committee of the HIRA in May, are at the end of drug price negotiations. Since Daewoong Pharmaceutical's Fexuclu, which passed the committee's review on the same day, released this month, Celltrion and Icure's Donpezil defeat are also expected to be listed soon. The Committee judged that the benefit was appropriate when accepting less than the evaluation amount, but the industry believes that the committee calculated the drug price lower than the pharmaceutical company's expectations. Developer Icure was also initially in a position to release the drug at a lower price than the original drug Aricept, but it is said that a lower drug price was suggested. The industry analyzes that if alternative drugs were included, they would fall short of the company's expectations. For example, the average price of the original and generic 10mg is 2,040 won, which is cheaper than the original Aricept 10mg (2,265 won) as well as the developer Icure's Penedo 10mg (2,460 won). Considering the compliance of dementia patients, it is analyzed that the use of patch drugs is better, so even if the drug price is low, it can record high performance due to its high usage. The market seems to be at a disadvantage for product developers because Donepezil prices are too low.
- Policy
- Hemophilia Type A tx, Bayer's Jivi has been approved
- by Lee, Hye-Kyung Jul 10, 2022 03:35pm
- Bayer's hemophilia type A treatment Jivi has been approved for domestic items. The MFDS recently granted Jivi to routine prophylaxis for on-demand and suppression of bleeding episodes in adults and adolescents (blood coagulation factor VIII birth deficiency) who have been treated before, management of bleeding before and after surgery, and reducing the frequency of bleeding episodes. Jivi obtained permission from the EU Commission in November of the same year after being approved by the U.S. FDA as a routine preventive treatment for youth and adult hemophilia patients aged 12 and older with a history of treatment in August 2018. Since t1/2 is maintained at 17.9 hours, the initial recommended dose is administered twice a week once every five days, and is administered every seven days or twice a week depending on the clinical characteristics of the patient. Depending on the bleeding episode, the frequency of administration can be further adjusted for each individual. The most common side effects were headaches, coughs, nausea, and fever. Hemophilia A treatments currently prescribed in Korea include Advate (Takeda), Novoseven (Novonordisc), GreenMono (GC Pharma), Xyntha Solofuse (Pfizer), Adynovate (Takeda), Greengene F (GC Pharma), Faiva (Korean Red Cross), There are about 10 products including Kogenate FS (Bayer), Immune (Korean Red Cross), Elocate (Sanopy), Hemlibra (JW Pharmaceutical), Kogenate FS (Bayer), Immunate (Korean Red Cross), Eloctate (Sanofi), and Hemlibra (JW Pharmaceutical). Hemophilia is largely divided into hemophilia A ( factor Ⅷ deficiency), hemophilia B ( factor Ⅸ deficiency), hemophilia C (factor XI deficiency), parahemophilia ( factor V deficiency), pseudohemophilia or von Wilbrand factor deficiency. The frequency of hemophilia A patients is about 1 out of every 5,000 to 10,000 normal born boys, and hemophilia B is about one-fifth of hemophilia A.I n the case of von Willebrand Disease, it occurs in both men and women, and it is about one in 1,000 people. It is estimated that there are 600,000 hemophilia patients around the world, and about 12% of all hemophilia patients are type B hemophilia. In Korea, hemophilia A has the largest number of patients with hemophilia at about 2,000. Hemophilia B patients are estimated to be 400 patients, von Wilbrand Disease patients are estimated to be 100 patients, and hemophilia C patients are estimated to be 20.
- Policy
- The MFDS announced the designation of 4 temporary narcotics
- by Lee, Hye-Kyung Jul 07, 2022 05:54am
- The MFDS (Director Oh Yoo-kyung) announced on the 5th that it will designate four types, including 1V-LSD, which are used as substitute substances for drugs. 1V-LSD is newly designated as Class I temporary drug, and CH-PIATA is newly designated as Class II temporary drug. 1V-LSD has a structure similar to that of LSD and is a material which may cause hallucinations and other effects. LSD is a powerful hallucinogenic agent that causes severe physical and mental dependence when misused. CH-PIATA is a synthetic hemp-related substance that has been confirmed to be distributed for misuse and abuse in Korea. Among the current Class II temporary narcotics, two types of Flubromazolam and Cumyl-4CN-B7AICA, which expire on September 9, will be re-designated as Class II temporary narcotics. The temporary narcotics designation system is to name substances that are abused as narcotics substitutes and may harm public health for up to three years. Since 2011, a total of 243 types have been designated by implementing a temporary drug designation system, and 150 types, including "THF-F," have been designated as narcotics after evaluating dependence. Substances designated as temporary drugs are handled and managed in the same manner as drugs from the date of designation notice. The substance is entirely prohibited from possession, possession, use, management, import and export, manufacture, sale, sale, mediation, receipt, etc., and can be seized. After being designated and announced as temporary drugs, the import, manufacture, sale, trade, mediation, or receipt of temporary drugs in Class I will be sentenced to life or imprisonment for more than five years. Exporting, importing, or manufacturing a Class II temporary narcotic will be punished with imprisonment for up to 10 years or a fine of 100 million won at maximum. Trading or brokering it will be punished with imprisonment for up to five years or a fine of 50 million won at maximum. The MFDS said it expects the announcement of new designation and re-designation of temporary drugs to block the distribution of new drugs and help public health. In addition, the MFDS said it will continue to do its best to protect public health from new and illegal drugs in cooperation with related agencies such as the prosecution, police, and customs offices.
- Policy
- Antiepileptic perampanel’s adverse event rate at 32%
- by Lee, Hye-Kyung Jul 05, 2022 05:59am
- Results of the 6-year post-marketing surveillance (PMS) on the antiepileptic ingredient perampanel showed that 17 cases of serious adverse reactions whose causal relationship cannot be ruled out were reported. As 287 cases of unexpected adverse reactions whose causal relationship cannot be ruled out were also reported, the indication for perampanel is set to be updated to account for these changes. The Ministry of Food and Drug Safety had announced that it had recently released an order (draft) to change the indication for 16 perampanel items owned by Eisai Korea, Whanin Pharmaceutical, and Myung In Pharm according to the reassessment results, and will be conducting an opinion inquiry on the proposed changes until the 14th. Eisai Korea received marketing authorization for its Fycompa Film Coated Tab on July 10th, 2015. Fycompa Film Coated Tab is indicated as monotherapy for the treatment of partial-onset seizures (POS) with or without secondarily generalized seizures in patients 4 years of age and older, and as adjunctive therapy for the treatment of primary generalized tonic-clonic (PGTC) seizures in patients 7 years of age and older with idiopathic generalized epilepsy (IGE). Results of the PMS that was conducted on 3,354 patients for 6 years for the reassessment in Korea showed that the rate of reported adverse events, regardless of casualties, was 32.62% (1094 /3354 subjects, 1376 cases in total). Among the reported adverse events, the rate of serious adverse reactions whose causal relationship cannot be ruled out was 0.51% (17/3354 subjects), and neurological disorders such as dizziness, mental disorders such as suicide attempts, as well as encephalitis, anemia, and spontaneous abortion were found to occur. Unexpected adverse reactions whose causal relationship cannot be ruled out such as memory impairment, cerebrovascular accidents, sleep disorders, fever, abdominal pain, eye pain, weight loss, erectile dysfunction, and palpitations were reported at a rate of 7.87% (264/354 subjects, 287 cases). Also, the rate of adverse events reported when perampanel is not administered according to the approved dosage and regimen was rather high, at 44% (85/193 subjects). The MFDS plans to change the indication for perampanel after conducting the opinion inquiry and issuing a preannouncement notice for the change order (draft).
- Policy
- The revaluation of Streptokinase could be maintained for 1yr
- by Lee, Tak-Sun Jul 05, 2022 05:59am
- The pharmaceutical industry expects some components to be suspended in this year's benefit revaluation. The benefit revaluation of Streptokinase and Streptodornase, which are scheduled to submit a report on clinical revaluation results next year, could be suspended for one year. However, the final plan is expected to be decided through deliberation by the Drug Benefit Evaluation Committee of the HIRA, which will be held on the 7th. An official from the pharmaceutical industry said on the 4th, "Streptokinase and Streptodornase, which are components subject to benefit reevaluation this year, are also discussing a one-year grace period." "However, I understand that it is not a confirmed issue," he explained. Streptokinase and Streptodornase are enzyme drugs such as Hanmi Pharmaceutical's Mucolase and SK Chemical's Varidase. Since 2017, the drug has been conducting clinical re-evaluation by the MFDS, and the previous indications of sinusitis and thrombosis have been deleted. Next year, it is scheduled to submit a report on the results of clinical re-evaluation of the difficulty of ventilation accompanied by respiratory diseases. Accordingly, the industry suggested that the re-evaluation of the drug should be suspended until the clinical re-evaluation of the drug is completed. This is because the cost of clinical trials that were in progress cannot be compensated. However, the HIRA said that benefit revaluation and clinical revaluation are different. On the 14th of last month, Kim Ae-ryeon, head of the HIRA's Pharmaceutical Price Benefit Division, explained, "The review of benefit adequacy may include the target of clinical revaluation by the MFDS by selecting the target according to the selection criteria." She said, "It is difficult to consider arbitrarily delaying the evaluation of benefit adequacy only for components subject to clinical revaluation in terms of equity with other components." It remains to be seen whether the re-evaluation of Streptokinase and Streptodronase salary will be postponed as the industry suggests. Industries are also predicting some re-evaluation results. In the case of Celltrion's liver disease solvent Godex, which has the highest benefit claim among the ingredients this year, it is uncertain whether it will be maintained, and on the contrary, the antacid Almagate is likely to be maintained. In the case of Eperisone HCl, pain muscle contraction accompanied by musculoskeletal disease, which is the first indication, is likely to maintain, but it is uncertain about stiffness paralysis due to nervous system disease, which is the second indication. However, another industry official said, "The HIRA maintains strict security this time, so little has been confirmed in the industry." "It is said that only two of the six target ingredients will be maintained," he said. Since the announcement of the benefit adequacy revaluation plan in March, The HIRA received data from the pharmaceutical company and conducted a practical review on whether each ingredient meets the evaluation criteria. Therefore, on the 7th, the results will be announced after the first deliberation by the Pharmaceutical Benefit Evaluation Committee with the results. After that, during the fourth quarter, the Post-Pharmaceutical Evaluation Committee and the Pharmaceutical Benefit Evaluation Committee will be held once more to deliberate on the final plan.
- Policy
- Support for online conferences will be extended for 1 yr
- by Kim, Jung-Ju Jul 05, 2022 05:58am
- On-off events can be supported on an offline basis if certain conditions are met Temporary support for online academic conferences in medical, pharmaceutical, and medical devices will be extended for another year. This is because infectious diseases such as COVID-19 and monkeypox continue to occur, leaving room for reinforcement of social distancing. However, in the case of online and offline parallel academic conferences, which are called hybrid, flexibility has been increased so that they can apply on an offline basis by setting a certain standard in a rigid method that was previously applied only on an online basis. Branches and online academic conferences held by individual nursing institutions, which have been evaluated as having poor support effects in the industry, will not be able to apply except for exception collection support. The Fair Trade Commission recently approved a one-year extension by partially revising the "temporary support extension plan for online academic conferences." The support for online academic conferences is the first extension in 2021 and the second extension this time since temporary approval was made due to the outbreak of COVID-19 in 2020. In response, the Fair Trade Commission explained, "Despite the end of the limited time period to support online academic conferences, we decided to extend the support period in consideration of the situation where non-face-to-face events will continue as COVID-19 becomes commonplace and the academic exchange environment changes." Subject to the support are academic conferences held by affiliated organizations, medical societies, and pharmacological branches under the articles of association of the Medical Association and the Hospital Association. It also includes academic societies (including overseas societies), academic institutions, organizations, research institutes, and organizations approved and recognized by doctors' associations, dental societies, oriental medicine societies, pharmacological societies, and herbal societies. The most noticeable change in this extension is the change in the existing policy that was supported online at both online and off-line events. If more than 20% of the total participants, including the speaker, attend offline academic conferences, they will be supported on an offline basis and up to two offline booths will be installed. In addition, the cost can be provided to the society by 2 million won to 3 million won per booth and 500,000 won to 1 million won for nursing institutions. However, in the case of academic conferences held by organizations affiliated with individual academic societies, branches, and individual nursing institutions, it was stipulated that they would not apply except for exception collection. The Fair Trade Commission and the MOHW decided to discuss again if the government's policies, such as re-strengthening social distancing, make it impossible to hold offline academic conferences.
- Policy
- Korea joined the COVID-19 vaccine development country
- by Lee, Hye-Kyung Jul 04, 2022 05:54am
- As SK Bioscience's COVID-19 vaccine SKY Covione received an item permit today (29th), Korea has become a country with both COVID-19 treatments and vaccines along with Celltrion's COVID-19 treatment Regkirona. Oh Yu-kyung, Minister of Food and Drug Safety announced on June 29 that it decided to grant item permission on condition that SKY Covione, a COVID-19 vaccine developed by SK Bioscience and applied for permission to manufacture, sell, and item, submit a final clinical trial result report. SKY Covione is a COVID-19 vaccine that induces an immune response by administering antigen proteins made using gene recombination technology. This product was licensed for the purpose of preventing COVID-19 in adults over the age of 18. For usage/dose, 0.5mL of an antigen vial mixed with the immune enhancer (AS03) is inoculated twice every four weeks. SKY Covione is a COVID-19 vaccine developed and manufactured by a domestic company, and is a hot topic as it is approved by the MFDS for the first time in the world. The MFDS operated vaccine projects to support the development of the domestic COVID-19 vaccine quickly amid the COVID-19 situation, intensively and systematically. Since September 2020, a permission review team consisting of reviewers with extensive experience in screening has been set up to support the development and commercialization of COVID-19 vaccines and treatments, and customized consultations and preliminary reviews by non-clinical, clinical, and quality stages. It supported the design of clinical trials by preemptively introducing an immunogenic comparative clinical trial method so that phase 3 clinical trials, which are a key stage of product development, can be carried out scientifically and quickly. It led discussions in meetings and workshops between regulatory agencies so that the immunogenic comparative clinical method could be recognized internationally, and reflected in the WHO guidelines published in March. Unlike the previously approved COVID-19 vaccine, the COVID-19 vaccine permit is also significant in that the MFDS evaluated safety, effectiveness and quality of the COVID-19 vaccine developed by Korean companies throughout the development stage. The MFDS held a final inspection committee at 10 a.m. on the 29th, which is the final screening stage for SKY Covione The MFDS received an application for SKY Covione on April 29, and the COVID-19 treatment and vaccine permission review team focused on data necessary for permission such as non-clinical, clinical, and quality. The clinical trial review evaluated safety and effectiveness through a total of two data, including one clinical trial (phase 1) conducted in Korea, one clinical trial (phase 3) conducted in six countries, Korea, the Philippines, Ukraine, Thailand, Vietnam, and New Zealand. In addition to reviewing data such as manufacturing methods, standards, and test methods, GMP implementation of domestic manufacturing plants were evaluated whether they had facilities and management systems that could consistently produce quality through on-site inspections. With the approval of SKY Covione, we can expect Korean companies to enter the global vaccine market in earnest in the future. SK Bioscience is pushing for the registration of the WHO Emergency Use List (EUL) and plans to prepare for the supply of vaccines through COVAX FACILITY. This licensed vaccine can be stored in a refrigerated storage (2 to 8℃), so even countries that do not have ultra-low temperature distribution equipment can expect effective use in quarantine. The MFDS said, "We launched a commercialization strategy support group to support rapid entry into the market of public health crisis drugs, new concepts, and new technology drugs, linking development, non-clinical, clinical trial, and licensing reviews, and strengthening the function of providing professional services for clinical trial design." It added that a total of 22.6 billion won (2022) was invested in building infrastructure through vaccine safety technology support centers, and that it is expected to have synergy effects in supporting drug development companies by promoting basic counseling on vaccine development, quality and clinical training. Oh Yu-kyung, Minister of Food and Drug Safety said, "The MFDS has thoroughly verified safety and effectiveness through a triple advisory process and approved SKY Covione of SK Bioscience." "In the future, we will work with various institutions to preemptively respond to future infectious diseases," she said.
- Policy
- Evusheld granted emergency use for preventing COVID-19
- by Lee, Hye-Kyung Jul 01, 2022 05:50am
- With the emergency use authorization has been granted for ‘Evusheld Inj,’ the first-ever preventive antibody therapy for COVID-19 in Korea, the drug is expected to be used in immunocompromised patients who are unlikely to mount an adequate response to COVID-19 vaccinations. The Ministry of Food and Drug Safety (Minister: Yu-Kyoung Oh) announced on the 30th that the ministry decided to grant emergency use authorization for 20,000 courses of the antibody therapy ‘Evusheld Inj (tixagevimab, cilgavimab) that was developed by AstraZeneca to prevent COVID-19 infections. The authorization was made after deliberation by the Medical Product Safety Management and Supply Committee for Public Health Emergency Response in comprehensive consideration of its need in immunocompromised patients that are unlikely to mount an adequate response to COVID-19 vaccinations, the MFDS’s safety, efficacy, and quality review results, and results from the expert advisory meeting. The Evusheld that received EUA today will become the first-ever antibody therapy authorized in Korea for COVID-19 prevention and is expected to contribute to the prevention of COVID-19 infections in blood cancer patients who may not mount an adequate immune response to COVID-19 vaccinations, as well as patients receiving immunosuppressant therapy after organ transplantation, etc. Evusheld has been granted emergency use authorization (EUA) in the US in December last year, then granted marketing authorization in Europe in March this year. Evusheld is a neutralizing antibody combination that binds to the spike protein of SARS-CoV-2 to inhibit virus penetration into bodies. Evusheld is approved for use in adults and adolescents (aged 12 and older who weigh 40kg or more) with moderate to severe immune compromise due to blood cancer, or immunosuppressive therapy after organ transplant that may not mount an adequate immune response to COVID-19 vaccination that are not currently infected with or had recent known exposure to a person infected with SARS-CoV-2. Evusheld is administered as an IM dose of tixagevimab (150㎎) and cilgavimab (150㎎) in two separate, consecutive injections. The emergency use authorization is a system that allows manufacturers and importers to supply unauthorized medical products in Korea to respond to a public health crisis. The Korea Disease Control and Prevention Agency requested for the EU A of Evusheld Inj. To the Ministry of Food and Drug Safety on June 10th this year. After the EUA, the MFDS will additionally make safety measures and collect information on adverse events arising from Evusheld’s use. The ministry also ordered its domestic importer to actively collect and report on its safety information in Korea and abroad, and established a system so that healthcare professionals and patients (and patient families) can report adverse events by phone or online. The ministry will continuously analyze and assess the safety information in Korea and abroad to promptly take safety measures when necessary.
- Policy
- Follow-up of impurity inspection
- by Lee, Hye-Kyung Jul 01, 2022 05:49am
- The MFDS received the results of testing and testing of Nitrosamine impurities from domestic pharmaceutical companies, and prepared safety management guidelines as a follow-up measure. This guideline is for safety management of N-Nitrosamines, a mutagenic and carcinogenic impurity among drugs, and it should be implemented by raw and finished drug manufacturers and importers to reduce or prevent impurities within the daily intake allowance. Starting with high blood pressure medicine (Valsartan) in 2018, tuberculosis medicine (Rifampicin) in 2021 as Nitrosamines were detected in drugs every year until Rifampicin), and new mutagenic impurities were recently detected, the MFDS received a test report of the company's own test on the evaluation items that may occur until the 31st of last month. The MFDS will continue to review impurities that may be included in medicines through close cooperation with overseas regulators and recommend appropriate measures to related pharmaceutical companies. According to the guidelines, apart from the MFDS' review of impurities, pharmaceutical companies should closely examine the raw material drug synthesis process and the manufacturing process of finished drugs using currently available science and technology to evaluate the potential cause and possibility of impurity generation. Pharmaceutical companies should check all impurities that may occur in the process of synthesizing and storing raw materials, manufacturing and storing finished drugs, evaluate the mutagenicity of the impurities, and if mutagenicity or carcinogenicity is confirmed, the risk of carcinogenesis is less than 1/100,000. (Q)SAR methods can be used to predict bacterial mutagenicity test results using a computer, appropriate management methods can be applied based on structural warnings, or bacterial mutagenicity tests can be conducted directly for specific impurities Nitrosamines impurities are compounds with potential carcinogenicity or mutagenicity that can occur mainly when they are combined under conditions where Amine and Nitrosating agent can react. The causes of Nitrosamines impurities in drugs identified so far can be classified as risk factors related to the manufacture and storage of raw and finished drugs, and there are also risk factors related to GMP. When detecting impurities, pharmaceutical companies should report data on the possibility of occurrence to the MFDS, the cause of occurrence (including the estimated cause), the detection amount, and the daily intake allowance. According to the step-by-step measures prepared and distributed by the MFDS, pharmaceutical companies should compare the amount of impurity detection and acceptance criteria, take necessary measures such as voluntary recovery and reduction of impurities in the market, and submit related matters to the MFDS. It will review necessary measures based on information collected from pharmaceutical companies' reporting of impurity detection and overseas regulatory agencies. If it is deemed necessary for the safety management of drugs in circulation in Korea, it is planned to instruct to evaluate the possibility of impurities and conduct test tests on specific drugs.